Gostatin
Topic: Chemistry
 From HandWiki - Reading time: 1 min
From HandWiki - Reading time: 1 min
| |
| Names | |
|---|---|
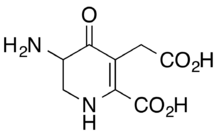
| IUPAC name
3-Amino-5-(carboxymethyl)-4-oxo-2,3-dihydro-1H-pyridine-6-carboxylic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H10N2O5 | |
| Molar mass | 214.177 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Gostatin is an irreversible inhibitor of the aspartate aminotransferase[1][2] produced by the bacterium Streptomyces sumanensis.[1][3] Its structure is a dihydro-4-pyridone analog of glutamic acid.
References
- ↑ 1.0 1.1 Lanthorn, T.H.; Fagg, G.E. (April 1989). "Gostatin blocks physiological actions and binding of acidic amino acids in rat brain". Neuropharmacology 28 (4): 429–432. doi:10.1016/0028-3908(89)90041-5. PMID 2546088.
- ↑ Coombs, Graham H. (7 October 1991) (in en). Biochemical Protozoology As A Basis For Drug Design. CRC Press. p. 115. ISBN 978-0-7484-0001-0.
- ↑ Meurant, Gerard (2 December 2012) (in en). Actinomycetes in Biotechnology. Elsevier. p. 304. ISBN 978-0-08-098433-9.
Further reading
- Friedman, Mendel (9 March 2013) (in en). Nutritional and Toxicological Significance of Enzyme Inhibitors in Foods. Springer Science & Business Media. p. 536. ISBN 978-1-4757-0022-0.
 |
Licensed under CC BY-SA 3.0 | Source: https://handwiki.org/wiki/Chemistry:Gostatin11 views | ↧ Download this article as ZWI file
 KSF
KSF