HIF prolyl-hydroxylase inhibitor
Topic: Chemistry
 From HandWiki - Reading time: 3 min
From HandWiki - Reading time: 3 min
| HIF prolyl-hydroxylase inhibitor | |
|---|---|
| Drug class | |
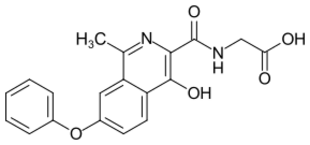 Roxadustat, the first marketed HIF prolyl-hydroxylase inhibitor | |
| Class identifiers | |
| ATC code | B03X |
| Mechanism of action | Enzyme inhibitor |
| Biological target | HIF prolyl-hydroxylase |
Not to be confused with Factor Inhibiting HIF Asparaginyl Hydroxylase Inhibitors
Hypoxia-inducible factor prolyl hydroxylase Inhibitors (HIF-PHIs) also known as hypoxia-inducible factor stabilizers (HIF stabilizers) are a novel class of drugs that act by inhibiting hypoxia-inducible factor-proline dioxygenase (HIF prolyl-hydroxylase) which is responsible for breaking down the hypoxia-inducible factor (HIF) under conditions of normal oxygen concentrations.
As of 2023, Vadadustat, Daprodustat, and Roxadustat are the most studied HIF-PHIs with highest number of phase III & Phase IV patient data for chronic kidney disease.[1][2] All the three drug are available in Japan, while Vadadustat & Daprodustat is under EU regulatory review for potential approval in 2023. US FDA approved Daprodustat in early 2023 after a positive adcom for favorable benefit-risk ratio, while Vadadustat is awaiting an even-handed response to its formal dispute resolution appeal, considering recent FDA approval of Daprodustat.[3]
Outside of chronic kidney disease, Akebia Therapeutics has reported initial findings from its phase II study evaluating Vadadustat for ARDS in Covid-19 patients.[4] Based on the results Akebia Therapeutics has decided to move forward with a phase III study in broader ARDS patients.[5]
The rights to Vadadustat is held by Akebia Therapeutics in partnership with CSL Vifor, Roxadustat by Fibrogen in partnership with Astellas, and Daprodustat has been internally developed by GSK.[6][7]
Examples
- Daprodustat
- Desidustat
- Enarodustat
- Molidustat
- Roxadustat (marketed in China)
- Vadadustat
See also
References
- ↑ Chen, Dinghua; Niu, Yue; Liu, Fei; Yang, Yue; Wang, Xue; Li, Ping; Chen, Xiangmei (2022-12-29). "Safety of HIF Prolyl-Hydroxylase Inhibitors for Anemia in Dialysis Patients: A Systematic Review and Network Meta-Analysis". Authorea Preprints. doi:10.22541/au.167229660.07194141. https://www.authorea.com/doi/full/10.22541/au.167229660.07194141.
- ↑ Zheng, Qiyan; Wang, Yahui; Yang, Huisheng; Sun, Luying; Zhang, Pingna; Zhang, Xueqin; Guo, Jing; Liu, Yu Ning et al. (2022-11-15). "Cardiac and Kidney Adverse Effects of HIF Prolyl-Hydroxylase Inhibitors for Anemia in Patients With CKD Not Receiving Dialysis: A Systematic Review and Meta-analysis" (in en). American Journal of Kidney Diseases 81 (4): 434–445.e1. doi:10.1053/j.ajkd.2022.09.014. ISSN 0272-6386. PMID 36396085. https://www.sciencedirect.com/science/article/pii/S0272638622010149.
- ↑ "HIF-PHI-delity? As FDA spins new tune in CKD anemia with GSK nod, others in class may hope to play along | BioWorld" (in en). https://www.bioworld.com/articles/693998-hif-phi-delity-as-fda-spins-new-tune-in-ckd-anemia-with-gsk-nod-others-in-class-may-hope-to-play-along,%20..
- ↑ Therapeutics, Akebia. "Akebia Therapeutics Announces Initial Findings from Investigator-Sponsored Clinical Study Evaluating Vadadustat for the Prevention and Treatment of Acute Respiratory Distress Syndrome (ARDS) in Subjects with COVID-19 and Hypoxemia (VSTAT)". www.prnewswire.com (Press release). Retrieved 2023-02-15.
- ↑ Transcripts, S. A. (2022-11-03). "Akebia Therapeutics, Inc. (AKBA) Q3 2022 Earnings Call Transcript | Seeking Alpha" (in en). https://seekingalpha.com/article/4552837-akebia-therapeutics-inc-akba-q3-2022-earnings-call-transcript.
- ↑ "vadadustat" (in en-GB). https://www.viforpharma.com/products/pipeline/vadadustat.
- ↑ "Astellas Receives European Commission Approval for First-in-Class EVRENZO™ (roxadustat) for Adult Patients with Symptomatic Anemia of Chronic Kidney Disease" (in en). https://newsroom.astellas.us/2021-08-19-Astellas-Receives-European-Commission-Approval-for-First-in-Class-EVRENZO-TM-roxadustat-for-Adult-Patients-with-Symptomatic-Anemia-of-Chronic-Kidney-Disease.
 |
 KSF
KSF