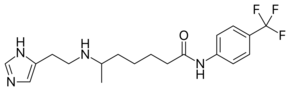
Histamine trifluoromethyl toluidide
Topic: Chemistry
 From HandWiki - Reading time: 4 min
From HandWiki - Reading time: 4 min
| |
| Names | |
|---|---|
| IUPAC name
6-[2-(1H-imidazol-5-yl)ethylamino]-N-[4-(trifluoromethyl)phenyl]heptanamide
| |
| Other names
HTFMT, HTMT
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C19H25F3N4O | |
| Molar mass | 382.42321 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Histamine trifluoromethyl toluidide (HTFMT) is a mixed H1/H2 histamine agonist which is significantly more potent than histamine itself.[1]
It also produces additional actions which appear to be independent of histamine receptors.[2][3]
References
- ↑ Whyment, AD; Blanks, AM; Lee, K; Renaud, LP; Spanswick, D (Apr 2006). "Histamine excites neonatal rat sympathetic preganglionic neurons in vitro via activation of H1 receptors". Journal of Neurophysiology 95 (4): 2492–500. doi:10.1152/jn.01135.2004. PMID 16354729.
- ↑ Qiu, R; Melmon, KL; Khan, MM (Jun 1990). "Effects of histamine-trifluoromethyl-toluidide derivative (HTMT) on intracellular calcium in human lymphocytes.". Journal of Pharmacology and Experimental Therapeutics 253 (3): 1245–52. doi:10.1016/S0022-3565(25)13228-X. PMID 2359026.
- ↑ Kim, DC; Lee, SY; Jun, DJ; Kim, SH; Lee, JH; Hur, EM; Baek, NI; Kim, KT (Nov 2005). "Inhibition of store-operated calcium entry-mediated superoxide generation by histamine trifluoromethyltoluide independent of histamine receptors". Biochemical Pharmacology 70 (11): 1613–22. doi:10.1016/j.bcp.2005.09.001. PMID 16219299.
 |
Licensed under CC BY-SA 3.0 | Source: https://handwiki.org/wiki/Chemistry:Histamine_trifluoromethyl_toluidide1 | ↧ Download this article as ZWI file
 KSF
KSF