Holmium acetylacetonate
Topic: Chemistry
 From HandWiki - Reading time: 4 min
From HandWiki - Reading time: 4 min
This article needs attention from an expert in chemicals. (October 2022) |
| |
| Names | |
|---|---|
| Other names
Holmium(III) acetylacetonate
| |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| C15H21HoO6 | |
| Molar mass | 462.257 g·mol−1 |
| Melting point | 101 °C (374 K)[1] |
| Insoluble[2] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Holmium acetylacetonate is a coordination complex, with the chemical formula of Ho(C5H7O2)3 or Ho(acac)3. It can be obtained via the reaction between metallic holmium[3] or holmium(III) hydride[1] with acetylacetone, or via the reaction between holmium(III) chloride and ammonium acetylacetonate.[4] Its anhydrous form is stable in a dry atmosphere but forms a hydrate in humid air.[5]
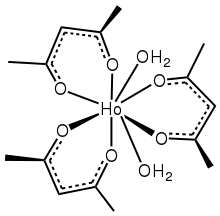
Like related lanthanide acetylacetonates, the complex is usually isolated with additional ligands. The dihydrate, which has been characterized by X-ray crystallography, features 8-coordinate Ho(III).[6] No crystallographic evidence has been reported for anhydrous derivatives.
References
- ↑ 1.0 1.1 Janice M. Koehler, William G. Bos (1967-12-01). "A novel synthesis of some anhydrous rare earth acetylacetonates" (in en). Inorganic and Nuclear Chemistry Letters 3 (12): 545–548. doi:10.1016/0020-1650(67)80023-0. https://linkinghub.elsevier.com/retrieve/pii/0020165067800230. Retrieved 2021-09-20.
- ↑ "Holmium Acetylacetonate" (in en). https://www.americanelements.com/holmium-acetylacetonate-14589-33-4.
- ↑ J.R. Blackborow, C.R. Eady, E.A.Koerner Von Gustorf, A. Scrivanti, O. Wolfbeis (1976-03-01). "Chemical syntheses with metal atoms" (in en). Journal of Organometallic Chemistry 108 (3): C32–C34. doi:10.1016/S0022-328X(00)92025-4. https://linkinghub.elsevier.com/retrieve/pii/S0022328X00920254. Retrieved 2021-09-20.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedZeisler - ↑ Trembovetskii, G. V.; Martynenko, L. I.; Murav'eva, I. A.; Spitsyn, V. Synthesis and study of volatile rare earth acetylacetonates. Doklady Akademii Nauk SSSR, 1984. 277 (6): 1411-1414. ISSN 0002-3264. In Russian.
- ↑ Kooijman, Huub; Nijsen, Frank; Spek, Anthony L.; Schip, Fred van het (2000). "Diaquatris(pentane-2,4-dionato-O,O′)holmium(III) monohydrate and diaquatris(pentane-2,4-dionato-O,O′)holmium(III) 4-hydroxypentan-2-one solvate dihydrate". Acta Crystallographica Section C Crystal Structure Communications 56 (2): 156–158. doi:10.1107/S0108270199013566. PMID 10777870.
External reading
- R. G. Bulgakov, S. P. Kuleshov, R. R. Vafin, A. G. Ibragimov, U. M. Dzhemilev (2008-03-01). "Reactions of lanthanide acetylacetonates with triethylaluminum" (in en). Kinetics and Catalysis 49 (2): 299–304. doi:10.1134/S0023158408020195. ISSN 0023-1584. http://link.springer.com/10.1134/S0023158408020195. Retrieved 2021-09-20.
- Kent J. Eisentraut, Robert E. Sievers (1967-08-01). "Thermogravimetric studies of metal β-diketonates" (in en). Journal of Inorganic and Nuclear Chemistry 29 (8): 1931–1936. doi:10.1016/0022-1902(67)80452-4. https://linkinghub.elsevier.com/retrieve/pii/0022190267804524. Retrieved 2021-09-20.
 |
Licensed under CC BY-SA 3.0 | Source: https://handwiki.org/wiki/Chemistry:Holmium_acetylacetonate7 views | ↧ Download this article as ZWI file
 KSF
KSF