Inorganic carbodiimide
Topic: Chemistry
 From HandWiki - Reading time: 3 min
From HandWiki - Reading time: 3 min

Inorganic carbodiimides (or cyanamides depending on the NCN2- form) design a family of compounds containing the carbodiimide (or cyanamide) anion NCN2- bonded to an inorganic group such as a metal. This anion exhibits pseudochalcogenide character.[1]
History
Adolph Frank and Nikodem Caro were the first to synthesize calcium cyanamide (CaNCN) and to develop a route for its production at the industrial level in 1898.[2] But, the very first beginning of the metal carbodiimides/cyanamides goes back to the 1870s. One can find detailed reports by E. Drechsel on the preparation of Ag2NCN, CaNCN, BaNCN, K2NCN and Li2NCN between 1875 and 1898 (before even XRD invention).[3][4] The preparation of Cd, Zn, Tl, Cu, Pb and Hg carboimides has been described during the 1950s. CaNCN is by the far the most known since it has been produced since 1898 and used as a fertilizer.
Starting from 2000s, rare earth metal carbodiimides Ln2(NCN)3 (Ln = La, Ce, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm and Tm)[5] and transition metal carbodiimides TNCN (T= Mn, Fe, Co, Ni, Cu) and Cr2(NCN)3have been reported.[6] Non-metallic carbodiimides are also known such as Si(NCN)2.[7]
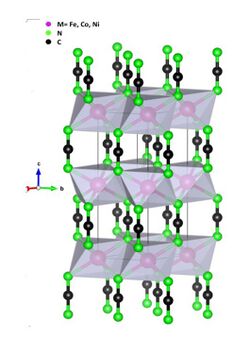
Structure
Tx(NCN)y binaries adopt generally structures with cationic layers made of octahedrally coordinated T2+/T3+ cations alternating with anionic layers of NCN2– in various structures such as rock-salt, NiAs- and NaN3-like structures. A side of binary carbodiimides, ternary (SrZn(NCN)2) and quaternary ones (Li2Sm2Sr(NCN)5 ) have also been prepared via solid-state metathesis.
Dimorphism is observed in inorganic carbodiimide. HgNCN for example can adopt two structures with two different NCN configurations, this means that HgNCN can have either carbodiimide[8] or cyanamide[9] structures.

Recently, metal carbodiimides are explored as anode materials for lithium and sodium ion batteries and found to present better specific capacities compared to conventional graphite and hard carbon electrode materials.[10][11]
References
- ↑ Corkett, Alex J.; Konze, Philipp M.; Dronskowski, Richard (2017-09-08). "The Ternary Post-transition Metal Carbodiimide SrZn(NCN)2". Zeitschrift für anorganische und allgemeine Chemie (Wiley) 643 (21): 1456–1461. doi:10.1002/zaac.201700225. ISSN 0044-2313.
- ↑ "Über ein neues Verfahren zur Erzeugung hoher Temperaturen und zur Darstellung von schwer schmelzbaren kohlefreien Metallen". Zeitschrift für Elektrotechnik und Elektrochemie 4 (21): 494–499. 1898-05-05. doi:10.1002/bbpc.18980042105. ISSN 0372-8323.
- ↑ Drechsel, Edmund (1875-05-10). "Beiträge zur Kenntniss des Cyanamids". Journal für Praktische Chemie 11 (1): 284–353. doi:10.1002/prac.18750110125. ISSN 0021-8383. https://zenodo.org/record/1427862.
- ↑ Drechsel, E. (1880-01-05). "Beiträge zur Kenntniss des Cyanamids;. II. Abhandlung". Journal für Praktische Chemie 21 (1): 77–97. doi:10.1002/prac.18800210104. ISSN 0021-8383.
- ↑ Neukirch, Michael; Tragl, Sonja; Meyer, H.-Jürgen (October 2006). "Syntheses and Structural Properties of Rare Earth Carbodiimides". Inorganic Chemistry 45 (20): 8188–8193. doi:10.1021/ic0608952. ISSN 0020-1669. PMID 16999417.
- ↑ Liu, Xiaohui; Krott, Manuel; Müller, Paul; Hu, Chunhua; Lueken, Heiko; Dronskowski, Richard (May 2005). "Synthesis, Crystal Structure, and Properties of MnNCN, the First Carbodiimide of a Magnetic Transition Metal†". Inorganic Chemistry 44 (9): 3001–3003. doi:10.1021/ic050050a. ISSN 0020-1669. PMID 15847401.
- ↑ Riedel, Ralf; Greiner, Axel; Miehe, Gerhard; Dressler, Wolfgang; Fuess, Hartmut; Bill, Joachim; Aldinger, Fritz (1997). "The First Crystalline Solids in the Ternary Si-C-N System". Angewandte Chemie International Edition in English 36 (6): 603–606. doi:10.1002/anie.199706031. ISSN 1521-3773.
- ↑ Liu, Xiaohui; Müller, Paul; Kroll, Peter; Dronskowski, Richard (2002-08-01). "Synthesis, Structure Determination, and Quantum-Chemical Characterization of an Alternate HgNCN Polymorph". Inorganic Chemistry 41 (16): 4259–4265. doi:10.1021/ic020133g. ISSN 0020-1669. PMID 12160416.
- ↑ Becker, M.; Jansen, M. (2000). "Synthese und Charakterisierung von Quecksilbercyanamid" (in de). Zeitschrift für anorganische und allgemeine Chemie 626 (7): 1639–1641. doi:10.1002/1521-3749(200007)626:7<1639::AID-ZAAC1639>3.0.CO;2-8. ISSN 1521-3749.
- ↑ Sougrati, Moulay T.; Darwiche, Ali; Liu, Xiaohiu; Mahmoud, Abdelfattah; Hermann, Raphael P.; Jouen, Samuel; Monconduit, Laure; Dronskowski, Richard et al. (2016). "Transition-Metal Carbodiimides as Molecular Negative Electrode Materials for Lithium- and Sodium-Ion Batteries with Excellent Cycling Properties". Angewandte Chemie International Edition 55 (16): 5090–5095. doi:10.1002/anie.201600098. ISSN 1521-3773. PMID 26989882.
- ↑ Dronskowski, Richard; Stievano, Lorenzo; Slabon, Adam; Mann, Markus; Liu, Xiaohui; Arayamparambil, Jeethu J.; Sougrati, Moulay T. (2018-08-14). "Carbodiimides as energy materials: which directions for a reasonable future?". Dalton Transactions 47 (32): 10827–10832. doi:10.1039/C8DT01846D. ISSN 1477-9234. PMID 30027198.
 |
 KSF
KSF