Iridium compounds
Topic: Chemistry
 From HandWiki - Reading time: 8 min
From HandWiki - Reading time: 8 min
| Oxidation states[lower-alpha 1] | |
|---|---|
| −3 | [Ir(CO)3]3− |
| −1 | [Ir(CO) 3(PPh 3)]1− |
| 0 | Ir 4(CO) 12 |
| +1 | [IrCl(CO)(PPh 3) 2] |
| +2 | Ir(C 5H 5) 2 |
| +3 | IrCl 3 |
| +4 | IrO 2 |
| +5 | Ir 4F 20 |
| +6 | IrF6 |
| +7 | [Ir(O 2)O 2]+ |
| +8 | IrO 4 |
| +9 | [IrO 4]+ [1] |
Iridium compounds are compounds containing the element iridium (Ir). Iridium forms compounds in oxidation states between −3 and +9, but the most common oxidation states are +1, +2, +3, and +4.[2] Well-characterized compounds containing iridium in the +6 oxidation state include IrF
6 and the oxides Sr
2MgIrO
6 and Sr
2CaIrO
6.[2][3] iridium(VIII) oxide (IrO
4) was generated under matrix isolation conditions at 6 K in argon.[4] The highest oxidation state (+9), which is also the highest recorded for any element, is found in gaseous [IrO
4]+
.[1]
Oxides
Only one binary oxide is well-characterized: Iridium dioxide, IrO2. It is a blue-black solid. The compound adopts the TiO2 rutile structure, featuring six coordinate iridium and three coordinate oxygen.[5] It adopts the fluorite structure.[2] A sesquioxide, Ir2O3, has been described as a blue-black powder, which is oxidized to IrO2 by HNO3.[6] The corresponding disulfides, diselenides, sesquisulfides, and sesquiselenides are known, as well as IrS3.[2]
Another oxide, iridium tetroxide, is also known, with iridium in the +8 oxiation state.[7] This compound was formed by photochemical rearrangement of [(η1-O2)IrO2] in solid argon at a temperature of 6 K (−267.15 °C; −448.87 °F). At higher temperatures, the oxide is unstable.[8] The detection of the iridium tetroxide cation IrO+4 by infrared photodissociation spectroscopy with formal oxidation state +9 has been reported, the highest currently known of any element, though the +10 oxidation state has been theorized for platinum, but not confirmed.[9][10]
Halides
Binary trihalides, IrX3 are known for all of the halogens.[2] For oxidation states +4 and above, only the tetrafluoride, pentafluoride and hexafluoride are known.[2] Iridium hexafluoride, IrF6, is a volatile yellow solid, composed of octahedral molecules. It decomposes in water and is reduced to IrF4,.[2] Iridium pentafluoride is also a strong oxidant, but it is a tetramer, Ir4F20, formed by four corner-sharing octahedra.[2]
Complexes

The coordination complexes of iridium are extensive.
Iridium in its complexes is always low-spin. Ir(III) and Ir(IV) generally form octahedral complexes.[2] Polyhydride complexes are known for the +5 and +3 oxidation states.[11] One example is IrH
5(PiPr
3)
2.[12] The ternary hydride Mg6Ir2H11 is believed to contain both the IrH4−5 and the 18-electron IrH5−4 anion.[13]
Iridium also oxyanions with oxidation states +4 and +5. K2IrO3 and KIrO3 can be prepared from the reaction of potassium oxide or potassium superoxide with iridium at high temperatures. Such solids are not soluble in conventional solvents.[14]
As for many elements, the chlorides are key complexes. Hexachloroiridic(IV) acid, H2IrCl6, and its ammonium salt are the most common iridium compounds from an industrial and preparative perspectives.[15] They are intermediates in the purification of iridium and used as precursors for most other iridium compounds, as well as in the preparation of anode coatings. The IrCl2−6 ion has an intense dark brown color, and can be readily reduced to the lighter-colored IrCl3−6 and vice versa.[15] Iridium trichloride, IrCl3, which can be obtained in anhydrous form from direct oxidation of iridium powder by chlorine at 650 °C,[15] or in hydrated form by dissolving Ir2O3 in hydrochloric acid, is often used as a starting material for the synthesis of other Ir(III) compounds.[2] Another compound used as a starting material is ammonium hexachloroiridate(III), (NH4)3IrCl6.
In the presence of air, iridium metal dissolves in molten alkali-metal cyanides to produce the Ir(CN)3−6 (hexacyanoiridate) ion.
Oxyanions
Iridium forms oxyanions in the +4 oxidation state. It forms compounds such as lithium iridate (Li2IrO3), which forms black crystals with three slightly different layered atomic structures, α, β, and sometimes γ. Lithium iridate exhibits metal-like, temperature-independent electrical conductivity,[17] and changes its magnetic ordering from paramagnetic to antiferromagnetic upon cooling to 15 K.[16] Lithium iridate is a potential electrode material for the lithium-ion battery.[17] This application is hindered by the high costs of Ir, as compared to the cheaper Li2MnO3 alternative.[18]
Organoiridium chemistry
Organoiridium compounds contain iridium–carbon bonds. Early studies identified the very stable tetrairidium dodecacarbonyl, Ir4(CO)12.[2] In this compound, each of the iridium atoms is bonded to the other three, forming a tetrahedral cluster. The discovery of Vaska's complex (IrCl(CO)[P(C6H5)3]2) opened the door for oxidative addition reactions, a process fundamental to useful reactions. For example, Crabtree's catalyst, a homogeneous catalyst for hydrogenation reactions.[19][20] Iridium is usually supplied commercially in the Ir(III) and Ir(IV) oxidation states. Important starting reagents being hydrated iridium trichloride and ammonium hexachloroiridate. These salts are reduced upon treatment with CO, hydrogen, and alkenes. Illustrative is the carbonylation of the trichloride:
- IrCl3(H2O)x + 3 CO → [Ir(CO)2Cl2]− + CO2 + 2 H+ + Cl− + (x-1) H2O
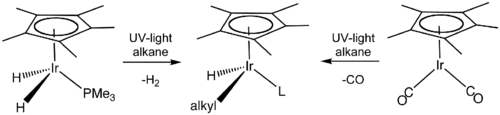
Many organoiridium(III) compounds are generated from pentamethylcyclopentadienyl iridium dichloride dimer. Many of derivatives feature kinetically inert cyclometalated ligands.[23] Related half-sandwich complexes were central in the development of C-H activation.[24][25]
Iridium complexes played a pivotal role in the development of carbon–hydrogen bond activation (C–H activation), which promises to allow functionalization of hydrocarbons, which are traditionally regarded as unreactive.[26]
See also
- Cobalt compounds
- Rhodium compounds
Notes
- ↑ Most common oxidation states of iridium are in bold. The right column lists one representative compound for each oxidation state.
References
- ↑ 1.0 1.1 Wang, Guanjun; Zhou, Mingfei; Goettel, James T.; Schrobilgen, Gary G.; Su, Jing; Li, Jun; Schlöder, Tobias; Riedel, Sebastian (2014). "Identification of an iridium-containing compound with a formal oxidation state of IX". Nature 514 (7523): 475–477. doi:10.1038/nature13795. PMID 25341786. Bibcode: 2014Natur.514..475W.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth–Heinemann. pp. 1113–1143, 1294. ISBN 978-0-7506-3365-9. OCLC 213025882.
- ↑ Jung, D.; Demazeau, Gérard (1995). "High Oxygen Pressure and the Preparation of New Iridium (VI) Oxides with Perovskite Structure: Sr 2MIrO 6 (M = Ca, Mg)". Journal of Solid State Chemistry 115 (2): 447–455. doi:10.1006/jssc.1995.1158. Bibcode: 1995JSSCh.115..447J.
- ↑ Gong, Y.; Zhou, M.; Kaupp, M.; Riedel, S. (2009). "Formation and Characterization of the Iridium Tetroxide Molecule with Iridium in the Oxidation State +VIII". Angewandte Chemie International Edition 48 (42): 7879–7883. doi:10.1002/anie.200902733. PMID 19593837.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Perry, D. L. (1995). Handbook of Inorganic Compounds. CRC Press. pp. 203–204. ISBN 978-1-4398-1461-1.
- ↑ Gong, Yu; Zhou, Mingfei; Kaupp, Martin; Riedel, Sebastian (2009). "Formation and Characterization of the Iridium Tetroxide Molecule with Iridium in the Oxidation State +VIII". Angewandte Chemie International Edition 48 (42): 7879–7883. doi:10.1002/anie.200902733. PMID 19593837.
- ↑ Citra, Angelo; Andrew, Lester (1999). "Reactions of Laser-Ablated Iridium Atoms with O2. Infrared Spectra and DFT Calculations for Iridium Dioxide and Peroxoiridium(VI) Dioxide in Solid Argon". J. Phys. Chem. A 103 (21): 4182–4190. doi:10.1021/jp990388o. Bibcode: 1999JPCA..103.4182C.
- ↑ Himmel, D.; Knapp, C.; Patzschke, M.; Riedel, S. (2010). "How far can we go? Quantum-chemical investigations of oxidation state IX". ChemPhysChem 11 (4): 865–869. doi:10.1002/cphc.200900910. PMID 20127784.
- ↑ Wang, Guanjun; Zhou, Mingfei; Goettel, James T.; Schrobilgen, Gary J.; Su, Jing; Li, Jun; Schlöder, Tobias; Riedel, Sebastian (23 October 2014). "Identification of an iridium-containing compound with a formal oxidation state of IX". Nature 514 (7523): 475–477. doi:10.1038/nature13795. PMID 25341786. Bibcode: 2014Natur.514..475W.
- ↑ Holleman, A. F.; Wiberg, E.; Wiberg, N. (2001). Inorganic Chemistry (1st ed.). Academic Press. ISBN 978-0-12-352651-9. OCLC 47901436.
- ↑ Esteruelas, Miguel A.; López, Ana M.; Oliván, Montserrat (2016). "Polyhydrides of Platinum Group Metals: Nonclassical Interactions and σ-Bond Activation Reactions". Chemical Reviews 116 (15): 8770–8847. doi:10.1021/acs.chemrev.6b00080. PMID 27268136.
- ↑ Černý, R.; Joubert, J.-M.; Kohlmann, H.; Yvon, K. (2002). "Mg 6Ir 2H 11, a new metal hydride containing saddle-like IrH5−4 and square-pyramidal IrH4−5 hydrido complexes". Journal of Alloys and Compounds 340 (1–2): 180–188. doi:10.1016/S0925-8388(02)00050-6.
- ↑ Gulliver, D. J.; Levason, W. (1982). "The chemistry of ruthenium, osmium, rhodium, iridium, palladium, and platinum in the higher oxidation states". Coordination Chemistry Reviews 46: 1–127. doi:10.1016/0010-8545(82)85001-7.
- ↑ 15.0 15.1 15.2 Renner, H.; Schlamp, G.; Kleinwächter, I.; Drost, E.; Lüschow, H. M.; Tews, P.; Panster, P.; Diehl, M. et al. (2002). "Platinum group metals and compounds". Ullmann's Encyclopedia of Industrial Chemistry. Wiley. doi:10.1002/14356007.a21_075. ISBN 978-3-527-30673-2.
- ↑ 16.0 16.1 16.2 Freund, F.; Williams, S. C.; Johnson, R. D.; Coldea, R.; Gegenwart, P.; Jesche, A. (2016). "Single crystal growth from separated educts and its application to lithium transition-metal oxides". Scientific Reports 6: 35362. doi:10.1038/srep35362. PMID 27748402. Bibcode: 2016NatSR...635362F.
- ↑ 17.0 17.1 O'Malley, Matthew J.; Verweij, Henk; Woodward, Patrick M. (2008). "Structure and properties of ordered Li2IrO3 and Li2PtO3". Journal of Solid State Chemistry 181 (8): 1803. doi:10.1016/j.jssc.2008.04.005. Bibcode: 2008JSSCh.181.1803O.
- ↑ Yoshio, Masaki; Brodd, Ralph J.; Kozawa, Akiya (17 July 2010). Lithium-Ion Batteries: Science and Technologies. Springer Science & Business Media. p. 10. ISBN 978-0-387-34445-4. https://books.google.com/books?id=gkYhDYk6ftQC&pg=PA10.
- ↑ Crabtree, R. H. (1979). "Iridium compounds in catalysis". Accounts of Chemical Research 12 (9): 331–337. doi:10.1021/ar50141a005.
- ↑ Crabtree, R. H. (2005). The Organometallic Chemistry of the Transition Metals. Wiley. ISBN 978-0-471-66256-3. OCLC 224478241. http://chimicibicocca.altervista.org/data/chimica_lucidi.pdf.
- ↑ Janowicz, A. H.; Bergman, R. G. (1982). "Carbon-hydrogen activation in completely saturated hydrocarbons: direct observation of M + R-H → M(R)(H)". Journal of the American Chemical Society 104 (1): 352–354. doi:10.1021/ja00365a091.
- ↑ Hoyano, J. K.; Graham, W. A. G. (1982). "Oxidative addition of the carbon-hydrogen bonds of neopentane and cyclohexane to a photochemically generated iridium(I) complex". Journal of the American Chemical Society 104 (13): 3723–3725. doi:10.1021/ja00377a032.
- ↑ Liu, Zhe; Sadler, Peter J. (2014). "Organoiridium Complexes: Anticancer Agents and Catalysts". Accounts of Chemical Research 47 (4): 1174–1185. doi:10.1021/ar400266c. PMID 24555658.
- ↑ Andrew H. Janowicz; Robert G. Bergman (1982). "Carbon–hydrogen activation in saturated hydrocarbons: direct observation of M + R−H → M(R)(H)". J. Am. Chem. Soc. 104: 352–354. doi:10.1021/ja00365a091.
- ↑ Graham, William A.G. (1982). "Oxidative addition of the carbon–hydrogen bonds of neopentane and cyclohexane to a photochemically generated iridium(I) complex". Journal of the American Chemical Society 104 (13): 3723–3725. doi:10.1021/ja00377a032.
- ↑ Hartwig, John F. (2011). "Regioselectivity of the Borylation of Alkanes and Arenes". Chemical Society Reviews 40 (4): 1992–2002. doi:10.1039/c0cs00156b. PMID 21336364.
 |
 KSF
KSF

