Isatoic anhydride
Topic: Chemistry
 From HandWiki - Reading time: 2 min
From HandWiki - Reading time: 2 min
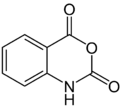
| |
| Names | |
|---|---|
| Preferred IUPAC name
2H-3,1-Benzoxazine-2,4(1H)-dione | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H5NO3 | |
| Molar mass | 163.132 g·mol−1 |
| Appearance | white solid |
| Melting point | 243 °C (469 °F; 516 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H317, H319 | |
| P261, P264+265Script error: No such module "Preview warning".Category:GHS errors, P272, P280, P302+352, P305+351+338, P321, P333+317Script error: No such module "Preview warning".Category:GHS errors, P337+317Script error: No such module "Preview warning".Category:GHS errors, P362+364Script error: No such module "Preview warning".Category:GHS errors, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Isatoic anhydride is an organic compound derived from anthranilic acid. A white solid, it is prepared by reaction of anthranilic acid with phosgene.[1]
Reactions
Hydrolysis gives carbon dioxide and anthranilic acid. Alcoholysis proceeds similarly, affording the ester:
- C6H4C2O3NH + ROH → C6H4(CO2R)(NH2) + CO2
Amines also effect ring-opening. Active methylene compounds and carbanions replace oxygen giving hydroxyquinolinone derivatives. Deprotonation followed by alkylation gives the N-substituted derivatives. Sodium azide gives the benzimidazolone via the isocyanate.[2] Isatoic anhydride is used as a blowing agent in the polymer industry, an application that exploits its tendency to release CO2.
Uses
Isatoic anhydride has been used as a precursor for the synthesis of methaqualone[3] and related 4-quinazolinone-based pharmaceutical drugs, including:
- Tioperidone
- Tranilast
- Pelanserin
- Diproqualone
- Antrafenine
- SJ-733
- Nicafenine
- Molinazone
- Cloperidone
- SGB 1534
References
- ↑ E. C. Wagner; Marion F. Fegley. (1947). "Isatoic anhydride". Org. Synth. 27: 45. doi:10.15227/orgsyn.027.0045.
- ↑ Coppola, Gary M. (1980). "The Chemistry of Isatoic Anhydride". Synthesis 7 (7): 505–36. doi:10.1055/s-1980-29110.
- ↑ Etienne F. van Zyl (2001). "A survey of reported syntheses of methaqualone and some positional and structural isomers". Forensic Sci. Int. 122 (2–3): 142–149. doi:10.1016/S0379-0738(01)00484-4. PMID 11672968.
 |
 KSF
KSF