Isoflavone
Topic: Chemistry
 From HandWiki - Reading time: 5 min
From HandWiki - Reading time: 5 min
Isoflavones are substituted derivatives of isoflavone, a type of naturally occurring isoflavonoids,[1][2] many of which act as phytoestrogens in mammals.[3] Isoflavones are produced almost exclusively by the members of the bean family, Fabaceae (Leguminosae). Although isoflavones and closely related phytoestrogens are sold as dietary supplements, there is little scientific evidence for either the safety of long-term supplementation or of health benefits from these compounds.[1] Some studies have identified potential risks from high intake of isoflavones, such as in women with a history of breast cancer, but this concern has not been substantiated with high-quality clinical research.[1]
Organic chemistry and biosynthesis
Isoflavone is an isomer of flavone, which is chromone substituted with a phenyl group in the 2-position. In isoflavone, the phenyl group is in the 3-position.[4]
Substituted isoflavone derivatives are related to the parent by the replacement of two or three hydrogen atoms with hydroxyl groups.[1]
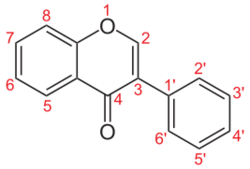
Isoflavone differs from flavone (2-phenyl-4H-1-benzopyr-4-one) in location of the phenyl group.
Isoflavones are produced via a branch of the general phenylpropanoid pathway that produces flavonoid compounds in higher plants. Soybeans are the most common source of isoflavones in human food; the major isoflavones in soybean are genistein and daidzein.[1] The phenylpropanoid pathway begins from the amino acid phenylalanine, and an intermediate of the pathway, naringenin, is sequentially converted into the isoflavone genistein by two legume-specific enzymes, isoflavone synthase, and a dehydratase. Similarly, another intermediate naringenin chalcone is converted to the isoflavone daidzein by sequential action of three legume-specific enzymes: chalcone reductase, type II chalcone isomerase, and isoflavone synthase. Plants use isoflavones and their derivatives as phytoalexin compounds to ward off disease-causing pathogenic fungi and other microbes. In addition, soybean uses isoflavones to stimulate soil-microbe rhizobium to form nitrogen-fixing root nodules.
Occurrence
Most members of the family Fabaceae contain significant quantities of isoflavones.[1] Analysis of levels in various species has found the highest levels of genistein and daidzein in psoralea (Psoralea corylifolia). Various legumes including soybean (Glycine max L.),[5] green bean (Phaseolus vulgaris L.), alfalfa sprout (Medicago sativa L.), mung bean sprout (Vigna radiata L.), cowpea (Vigna unguiculata L.), kudzu root (Pueraria lobata L.), and red clover blossom and red clover sprout (Trifolium pratense L.) have been studied for their estrogenic activity.[6] Highly processed foods made from legumes, such as tofu, retain most of their isoflavone content, and fermented miso, which has increased levels.
Soy milk has a much higher concentration of isoflavones than soy sauce, but fermented soybeans show considerably higher concentrations, with tempeh having the highest isoflavone content.[1][7]
Other dietary sources of isoflavones include chick pea (biochanin A), alfalfa (formononetin), and peanut (genistein). Isoflavones are also found in foods of animal origin such as dairy products,[8] meat, eggs and seafood,[9] but the overall contribution to total intake is low. In countries using the chorleywood bread process, such as in the UK, bread is a source of isoflavones from soy.[10]
In plant tissue, they most often occur as glycosides or their respective malonates or acetyl conjugates,[5] rendering them even more water-soluble (see isoflavone-7-O-beta-glucoside 6"-O-malonyltransferase). The latter forms are unstable and are transformed, e.g. by decarboxylation. Often when leguminose plants are challenged with viral or fungal infections, the water-soluble transport forms are hydrolyzed to the respective aglycones at the target site.[11]
Research
The consumption of isoflavones-rich food or dietary supplements is under preliminary research for its potential association with lower rates of postmenopausal cancer and osteoporosis in women.[1][12][13] Use of soy isoflavone dietary supplements may be associated with reduction of hot flashes in postmenopausal women.[1][12] Soy isoflavones can act as substrates for thyroid peroxidase, thereby competitively inhibiting thyroid hormone production.[14]
Despite the frequent use of isoflavone supplements, there are insufficient data on safety and adverse effects.[1] Isoflavones have GRAS status in the United States.[15] In a risk assessment of isoflavone supplements for post-menopausal women, the European Food Safety Authority found no adverse effects with intakes up to 150 mg/d, although it criticized the lack of data.[16]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 "Soy isoflavones". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis. 2016. http://lpi.oregonstate.edu/mic/dietary-factors/phytochemicals/soy-isoflavones.
- ↑ "A comparative survey of leguminous plants as sources of the isoflavones, genistein and daidzein: implications for human nutrition and health". J Altern Complement Med 3 (1): 7–12. 1997. doi:10.1089/acm.1997.3.7. PMID 9395689.
- ↑ Heber, D (2008). "Plant Foods and PhyTOChemicals in Human Health". in Berdanier, C.D. Handbook of Nutrition and Food, Second Edition. CRC Press. pp. 176–181. doi:10.1201/9781420008890.ch70. ISBN 978-0-8493-9218-4.
- ↑ Gaspar, Alexandra; Matos, Maria João; Garrido, Jorge; Uriarte, Eugenio; Borges, Fernanda (2014). "Chromone: A Valid Scaffold in Medicinal Chemistry". Chemical Reviews 114 (9): 4960–4992. doi:10.1021/cr400265z. PMID 24555663. https://repositorio-aberto.up.pt/handle/10216/70808.
- ↑ 5.0 5.1 Cotrim, G. S.; Silva, D. M.; Graça, J. P.; Oliveira Junior, A.; Castro, C.; Zocolo, G. J.; Lannes, L. S.; Hoffmann-Campo, C. B. (2023). "Glycine max (L.) Merr. (Soybean) metabolome responses to potassium availability" (in en). Phytochemistry 205: 113472. doi:10.1016/j.phytochem.2022.113472. ISSN 0031-9422. PMID 36270412. https://www.sciencedirect.com/science/article/pii/S0031942222003880.
- ↑ Boué, Stephen M.; Wiese, Thomas E.; Nehls, Suzanne; Burow, Matthew E.; Elliott, Steven; Carter-Wientjes, Carol H.; Shih, Betty Y.; McLachlan, John A. et al. (2003). "Evaluation of the Estrogenic Effects of Legume Extracts Containing Phytoestrogens". Journal of Agricultural and Food Chemistry 51 (8): 2193–2199. doi:10.1021/jf021114s. ISSN 0021-8561. PMID 12670155.
- ↑ "Bioactivity of dietary polyphenols: The role of metabolites". Critical Reviews in Food Science and Nutrition 60 (4): 626–659. 2020. doi:10.1080/10408398.2018.1546669. PMID 30614249.
- ↑ Kasparovska, J; Pecinkova, M; Dadakova, K; Krizova, L; Hadrova, S; Lexa, M; Lochman, J; Kasparovsky, T (2016). "Effects of Isoflavone-Enriched Feed on the Rumen Microbiota in Dairy Cows". PLOS ONE 11 (4): e0154642. doi:10.1371/journal.pone.0154642. PMID 27124615. Bibcode: 2016PLoSO..1154642K.
- ↑ Kuhnle, G. G.; Dell'Aquila, C; Aspinall, S. M.; Runswick, S. A.; Mulligan, A. A.; Bingham, S. A. (2008). "Phytoestrogen content of foods of animal origin: Dairy products, eggs, meat, fish, and seafood". Journal of Agricultural and Food Chemistry 56 (21): 10099–104. doi:10.1021/jf801344x. PMID 18922017.
- ↑ Mulligan, A. A.; Welch, A. A.; McTaggart, A. A.; Bhaniani, A; Bingham, S. A. (2007). "Intakes and sources of soya foods and isoflavones in a UK population cohort study (EPIC-Norfolk)". European Journal of Clinical Nutrition 61 (2): 248–54. doi:10.1038/sj.ejcn.1602509. PMID 16943849.
- ↑ Long-ze Lin (2000). "LC-ESI-MS Study of the Flavonoid Glycoside Malonates of Red Clover (Trifolium pratense)". Journal of Agricultural and Food Chemistry 2 (48): 354–365. doi:10.1021/jf991002. PMID 10691640.
- ↑ 12.0 12.1 "Soy". MedlinePlus, US National Library of Medicine. 30 April 2013. https://www.nlm.nih.gov/medlineplus/ency/article/007204.htm.
- ↑ Wei, P; Liu, M; Chen, Y; Chen, D. C. (2012). "Systematic review of soy isoflavone supplements on osteoporosis in women". Asian Pacific Journal of Tropical Medicine 5 (3): 243–8. doi:10.1016/S1995-7645(12)60033-9. PMID 22305793.
- ↑ "Effects of isoflavones on breast tissue and the thyroid hormone system in humans: a comprehensive safety evaluation". Archives of Toxicology 92 (9): 2703–2748. 2018. doi:10.1007/s00204-018-2279-8. PMID 30132047.
- ↑ Archer Daniels Midland Company (4 February 1998). "GRAS Notification for Isoflavones Derived from Soybeans". US Food and Drug Administration. https://www.fda.gov/downloads/food/ingredientspackaginglabeling/gras/noticeinventory/ucm264301.pdf.
- ↑ EFSA panel on food additives and nutrient sources added to food (21 October 2015). "Risk assessment for peri- and post-menopausal women taking food supplements containing isolated isoflavones". EFSA Journal 13 (10): 4246. doi:10.2903/j.efsa.2015.4246. http://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/4246.pdf. Retrieved 25 July 2016.
 |
 KSF
KSF