Josiphos ligands
Topic: Chemistry
 From HandWiki - Reading time: 5 min
From HandWiki - Reading time: 5 min

A Josiphos ligand is a type of chiral diphosphine which has been modified to be substrate-specific; they are widely used for enantioselective synthesis.[2] They are widely used in asymmetric catalysis.[3]
History

Modern enantioselective synthesis typically applies a well-chosen homogeneous catalyst for key steps. The ligands on these catalysts confer chirality. The Josiphos family of privileged ligands provides especially high yields in enantioselective synthesis.[4][5]
In the early 1990s, Antonio Togni began studying at the Ciba (now Novartis) Central Research Laboratories[6] previously-known[7] ferrocenyl ligands for a Au(I)-catalyzed aldol reaction.[6] Togni's team began considering diphosphine ligands, and technician Josi Puleo prepared the first ligands with secondary phosphines. The team applied Puleo's products in an Ru-catalyzed enamide hydrogenation synthesis; in a dramatic success, the reaction had e.e. >99% and a turnover frequency (TOF) 0.3 s−1.[6][7] The same ligand proved useful in production of (S)-metolachlor, active ingredient in the most common herbicide in the United States. Synthesis requires enantioselective hydrogenation of an imine; after introduction of the catalyst, the reaction proceeds with 100% conversion, turnover number (TON) >7mil, and turnover frequency >0.5 ms−1. This process is the largest-scale application of enantioselective hydrogenation, producing over 10 kilotons/year of the desired product with 79% e.e.[2] [1]
Josiphos ligands also serve in non-enantioselective reactions: a Pd-catalyzed reaction of aryl chlorides and aryl vinyl tosylates with TON of 20,000 or higher,[8] catalytic carbonylation,[9] or Grignard and Negishi couplings[10][11] A variety of Josiphos ligands are commercially available under licence from Solvias. The (R-S) and its enantiomer provide higher yields and enantioselectivities than the diastereomer (R,R).[1]
The ferrocene scaffold has proved to be versatile.[12] [13][14]
The consensus for the naming is abbreviating the individual ligand as (R)-(S)-R2PF-PR'2. The substituent on the Cp is written in front of the F and the R on the chiral center after the F.[2]
Reactions using Josiphos ligands
Some reactions that are accomplished using M-Josiphos complexes as catalyst are listed below. Other reactions where Josiphos ligands can be used are: hydrogenation of C=N, C=C and C=O bonds, catalyzed allylic substitution, hydrocarboxylation, Michael addition, allylic alkylation, Heck-type reactions, oxabicycle ring-opening, and allylamine isomerization. ; Hydroboration of styrene
- File:HB of styrene.png
- Conducted at -78 °C, the above reaction has e.e.'s up to 92% and TOF of 5-10 h−1.[15] Hayashi's Rh-binap complex gives better yield.[16]
- Hydroformylation of Styrene
- File:Hydroformylation of styrene.png
- This reaction scheme yields of up to 78% ee of the (R) product, but low TON and TOF of 10-210 and 1-14h−1 (respectively).[2][17]
- Reductive amination
- File:Amination of s metolachlor.png
- Above is the preparation of (S)-metolachlor. Good yields and a 100% conversion crucially require AcOH solvent.[16]
- Hydrogenation of exocyclic methyl imine
- File:Exocyclic imine hydrogenation.png
- This key step to synthesize a HIV integrase inhibitor, Crixivan, is one of the few known homogeneous heteroarene hydrogenation reactions. Bulky R groups increase the catalyst's performance, with 97% e.e. and TON and TOF of 1k and 8 min−1, respectively.[18][19]
- Asymmetric synthesis of chromanoylpyridine derivatives
- File:HIV rxn.png
- This reaction, for an intermediate in synthesis of an antihypertensive and anti-alopecic chromanoylpyridine derivative, exhibits high enantioselectivity, but low activity.[20]
Modified Josiphos ligands
Many variations of Josiphos ligands have been reported. One family is prepared from Ugi's amine.
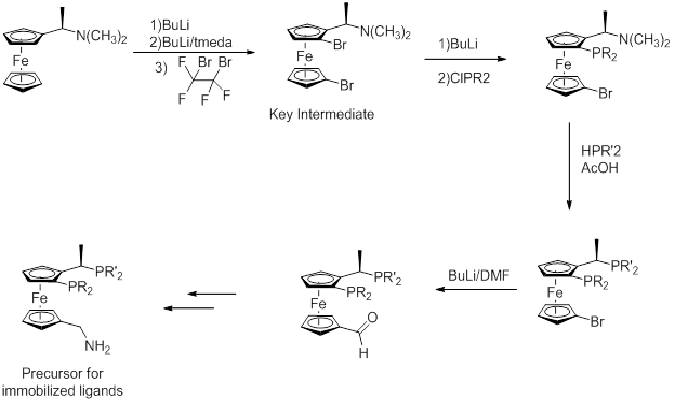
An important improvement on initial syntheses has been using N(CH3)2 as a leaving group over acetate, although an acetic acid solvent gives better yields.[6]
Further reading
Clevenger, Andrew L.; Stolley, Ryan M.; Aderibigbe, Justis; Louie, Janis (2020). "Trends in the Usage of Bidentate Phosphines as Ligands in Nickel Catalysis". Chemical Reviews 120 (13): 6124–6196. doi:10.1021/acs.chemrev.9b00682. PMID 32491839.
References
- ↑ 1.0 1.1 1.2 1.3 Blaser, Hans Ulrich; Pugin, Benoît; Spindler, Felix (2021). "Having Fun (And Commercial Success) with Josiphos and Related Chiral Ferrocene Based Ligands". Helvetica Chimica Acta 104. doi:10.1002/hlca.202000192.
- ↑ 2.0 2.1 2.2 2.3 Blaser, Hans-Ulrich; Brieden, Walter; Pugin, Benoit; Spindler, Felix; Studer, Martin; Togni, Antonio (2002). "Solvias Josiphos Ligands: From Discovery to Technical Applications". Topics in Catalysis 19 (1): 3–16. doi:10.1023/A:1013832630565. http://link.springer.com/10.1023/A:1013832630565.
- ↑ Blaser, Hans-Ulrich; Pugin, Benoît; Spindler, Felix; Mejía, Esteban; Togni, Antonio (2011-04-06). Zhou, Qi-Lin. ed (in en). Josiphos Ligands: From Discovery to Technical Applications (1 ed.). Wiley. pp. 93–136. doi:10.1002/9783527635207.ch3. ISBN 978-3-527-32704-1. https://onlinelibrary.wiley.com/doi/10.1002/9783527635207.ch3.
- ↑ Spessard, Gary O.; Miessler, Gary L. (2010). Organometallic chemistry (2 ed.). New York: Oxford University Press. pp. 378–379. ISBN 978-0-19-533099-1.
- ↑ Elschenbroich, Christopher (2006). Organometallics: Third Edition. pp.518-519
- ↑ 6.0 6.1 6.2 6.3 Togni, Antonio (1996-03-27). "Developing New Chiral Ferrocenyl Ligands for Asymmetric Catalysis: A Personal Account". CHIMIA 50 (3): 86. doi:10.2533/chimia.1996.86. ISSN 2673-2424. https://chimia.ch/chimia/article/view/1996_086.
- ↑ 7.0 7.1 Ito, Yoshihiko.; Sawamura, Masaya.; Hayashi, Tamio. (October 1986). "Catalytic asymmetric aldol reaction: reaction of aldehydes with isocyanoacetate catalyzed by a chiral ferrocenylphosphine-gold(I) complex" (in en). Journal of the American Chemical Society 108 (20): 6405–6406. doi:10.1021/ja00280a056. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja00280a056.
- ↑ Littke, Adam F.; Fu, Gregory C. (2002-11-15). "Palladium-Catalyzed Coupling Reactions of Aryl Chlorides". Angewandte Chemie International Edition 41 (22): 4176–4211. doi:10.1002/1521-3773(20021115)41:22<4176::AID-ANIE4176>3.0.CO;2-U. PMID 12434342. https://onlinelibrary.wiley.com/doi/10.1002/1521-3773(20021115)41:223.0.CO;2-U.
- ↑ Cai, Chaoxian; Rivera, Nelo R.; Balsells, Jaume; Sidler, Rick R.; McWilliams, J. Christopher; Shultz, C. Scott; Sun, Yongkui (2006-10-01). "An Efficient Catalyst for Pd-Catalyzed Carbonylation of Aryl Arenesulfonates" (in en). Organic Letters 8 (22): 5161–5164. doi:10.1021/ol062208g. ISSN 1523-7060. PMID 17048868. https://pubs.acs.org/doi/10.1021/ol062208g.
- ↑ Limmert, Michael E.; Roy, Amy H.; Hartwig, John F. (2005-11-01). "Kumada Coupling of Aryl and Vinyl Tosylates under Mild Conditions" (in en). The Journal of Organic Chemistry 70 (23): 9364–9370. doi:10.1021/jo051394l. ISSN 0022-3263. PMID 16268609. https://pubs.acs.org/doi/10.1021/jo051394l.
- ↑ Vo, Giang D.; Hartwig, John F. (2009-08-12). "Palladium-Catalyzed Coupling of Ammonia with Aryl Chlorides, Bromides, Iodides, and Sulfonates: A General Method for the Preparation of Primary Arylamines" (in en). Journal of the American Chemical Society 131 (31): 11049–11061. doi:10.1021/ja903049z. ISSN 0002-7863. PMID 19591470.
- ↑ Colacot, Thomas J. (2003). "A Concise Update on the Applications of Chiral Ferrocenyl Phosphines in Homogeneous Catalysis Leading to Organic Synthesis". Chemical Reviews 103 (8): 3101–3118. doi:10.1021/cr000427o. PMID 12914493.
- ↑ Blaser, Hans-Ulrich; Malan, Christophe; Pugin, Benoît; Spindler, Felix; Steiner, Heinz; Studer, Martin (January 2003). "Selective Hydrogenation for Fine Chemicals: Recent Trends and New Developments" (in en). Advanced Synthesis & Catalysis 345 (1–2): 103–151. doi:10.1002/adsc.200390000. ISSN 1615-4150. https://onlinelibrary.wiley.com/doi/10.1002/adsc.200390000.
- ↑ Chen, W. and Blaser, H.U 2008 in Phosphorus Ligands in Asymmetric Catalysis: Synthesis and Applications. (e.d. A. Borner) pp. 359-393
- ↑ T. Hayashi, Comprehensive Asymmetric Catalyst, eds. E.N. Jacobsen, A. Pfaltz and H. Yamamoto, 1999 pp. 247
- ↑ 16.0 16.1 Blaser, Hans-Ulrich; Buser, Hans-Peter; Jalett, Hans-Peter; Pugin, Benoit; Spindler, Felix (1999-12-31). "Iridium Ferrocenyl Diphosphine Catalyzed Enantioselective Reductive Alkylation of a Hindered Aniline" (in en). Synlett 1999 (Sup. 1): 867–868. doi:10.1055/s-1999-3106. ISSN 0936-5214. http://www.thieme-connect.de/DOI/DOI?10.1055/s-1999-3106.
- ↑ Godard, Cyril; Ruiz, Aurora; Claver, Carmen (August 2006). "Systematic Study of the Asymmetric Methoxycarbonylation of Styrene Catalyzed by Palladium Systems Containing Chiral Ferrocenyl Diphosphine Ligands" (in en). Helvetica Chimica Acta 89 (8): 1610–1622. doi:10.1002/hlca.200690161. ISSN 0018-019X. https://onlinelibrary.wiley.com/doi/10.1002/hlca.200690161.
- ↑ R.Fuchs, EP 803502(1996) assigned to Lonza A.G
- ↑ Studer, Martin; Wedemeyer-Exl, Christina; Spindler, Felix; Blaser, Hans-Ulrich (2000-12-13). "Enantioselective Homogeneous Hydrogenation of Monosubstituted Pyridines and Furans". Monatshefte fuer Chemie/Chemical Monthly 131 (12): 1335–1343. doi:10.1007/s007060070013. http://link.springer.com/10.1007/s007060070013.
- ↑ E. Broger, Y. Crameri and P. Jones, WO 99/01 453. (1997), assigned to Hoffman-La Roche
 |
 KSF
KSF