Lactam
Topic: Chemistry
 From HandWiki - Reading time: 3 min
From HandWiki - Reading time: 3 min

A lactam is a cyclic amide, formally derived from an amino alkanoic acid through cyclization reactions. The term is a portmanteau of the words lactone + amide.
Nomenclature
Greek prefixes in alphabetical order indicate ring size.
| Ring size (number of atoms in the ring) |
Systematic name | IUPAC name | Common name(s) | Structure |
|---|---|---|---|---|
| 3 | α-Lactam | Aziridin-2-one | α-Acetolactam | 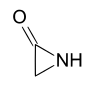 |
| 4 | β-Lactam | Azetidin-2-one | β-Propiolactam | 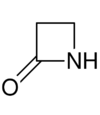 |
| 5 | γ-Lactam | Pyrrolidin-2-one |
|
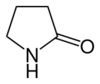 |
| 6 | δ-Lactam | Piperidin-2-one |
|
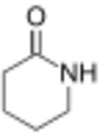 |
| 7 | ε-Lactam | Azepan-2-one |
|
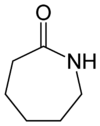 |
This ring-size nomenclature stems from the fact that hydrolysis of an α-lactam gives an α-amino acid and that of a β-Lactam gives a β-amino acid, and so on.
Synthesis
General synthetic methods are used for the organic synthesis of lactams.
Beckmann rearrangement
Lactams form by the acid-catalyzed rearrangement of oximes in the Beckmann rearrangement.
Schmidt reaction
Lactams form from cyclic ketones and hydrazoic acid in the Schmidt reaction.
Cyclization of amino acids
Lactams can be formed from cyclisation of amino acids via the coupling between an amine and a carboxylic acid within the same molecule. Lactamization is most efficient in this way if the product is a γ-lactam. For example, Fmoc-Dab(Mtt)-OH, although its side-chain amine is sterically protected by extremely bulky 4-Methyltrityl (Mtt) group, the amine can still intramolecularly couple with the carboxylic acid to form a γ-lactam. This reaction almost finished within 5 minutes with many coupling reagents (e.g. HATU and PyAOP).[1]
Intramolecular nucleophilic substitution
Lactams form from intramolecular attack of linear acyl derivatives from the nucleophilic abstraction reaction.
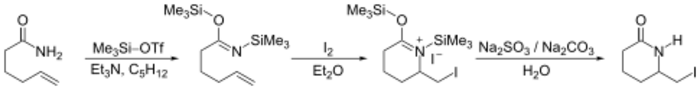
Iodolactamization
An iminium ion reacts with a halonium ion formed in situ by reaction of an alkene with iodine.[2]
Kinugasa reaction
Lactams form by copper-catalyzed 1,3-dipolar cycloaddition of alkynes and nitrones in the Kinugasa reaction
Diels-Alder reaction
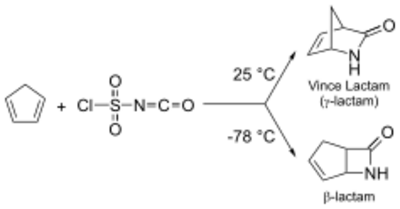
Diels-Alder reaction between cyclopentadiene and chlorosulfonyl isocyanate (CSI) can be utilized to obtain both β- as well as γ-lactam. At lower temp (−78 °C), β-lactam is the preferred product. At optimum temperatures, a highly useful γ-lactam known as Vince Lactam[3] is obtained.[4]
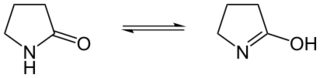
Lactam–lactim tautomerism
A lactim is a cyclic imidic acid compound characterized by an endocyclic carbon-nitrogen double bond. They are formed when lactams undergo tautomerization.
Reactions
- Lactams can polymerize to polyamides.
See also
- Lactone, a cyclic ester.
- β-Lactam
- β-Lactam antibiotics, which includes penicillins
- 2-Pyrrolidone
- 2-Piperidinone
- Caprolactam
References
- ↑ Lam, Pak-Lun; Wu, Yue; Wong, Ka-Leung (30 March 2022). "Incorporation of Fmoc-Dab(Mtt)-OH during solid-phase peptide synthesis: a word of caution" (in en). Organic & Biomolecular Chemistry 20 (13): 2601–2604. doi:10.1039/D2OB00070A. ISSN 1477-0539. PMID 35258068. https://pubs.rsc.org/en/content/articlelanding/2022/ob/d2ob00070a.
- ↑ Spencer Knapp, Frank S. Gibson Organic Syntheses, Coll. Vol. 9, p.516 (1998); Vol. 70, p.101 (1992) Online article
- ↑ Singh, R.; Vince, R. Chem. Rev. 2012, 112 (8), pp 4642–4686."2-Azabicyclo[2.2.1]hept-5-en-3-one: Chemical Profile of a Versatile Synthetic Building Block and its Impact on the Development of Therapeutics"
- ↑ Pham, P.-T.; Vince, R. Phosphorus, Sulphur and Silicon 2007, 779-791.
External links
 |
 KSF
KSF