Lanthanum trifluoride
Topic: Chemistry
 From HandWiki - Reading time: 6 min
From HandWiki - Reading time: 6 min
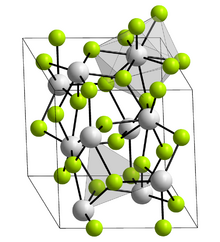 Crystal structure
| |
| Names | |
|---|---|
| Other names
Lanthanum(III) fluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| LaF3 | |
| Molar mass | 195.900 g/mol[1] |
| Appearance | white, crystalline solid |
| Density | 5.9 g/cm3[1] |
| Melting point | 1,493 °C (2,719 °F; 1,766 K)[1] |
Refractive index (nD)
|
1.606 |
| Structure | |
| Rhombohedral, hR24 | |
| P3c1, No. 165[2] | |
a = 0.7185 nm, c = 0.7351 nm
| |
Lattice volume (V)
|
0.32865 |
Formula units (Z)
|
6 |
| Hazards | |
| Safety data sheet | [3] |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions
|
Lanthanum(III) chloride Lanthanum(III) bromide Lanthanum(III) iodide |
Other cations
|
Actinium(III) fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lanthanum trifluoride is a refractory ionic compound of lanthanum and fluorine.[4] The chemical formula is LaF3.
The LaF3 structure
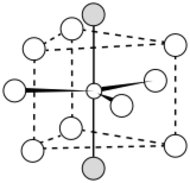
Bonding is ionic with lanthanum highly coordinated. The cation sits at the center of a trigonal prism. Nine fluorine atoms are close: three at the bottom corners of the trigonal prism, three in the faces of the trigonal prism, and three at top corners of the trigonal prism. There are also two fluorides a little further away above and below the prism. The cation can be considered 9-coordinate or 11-coordinate.[4] At 300 K, the structure allows the formation of Schottky defects with an activation energy of 0.07 eV, and free flow of fluoride ions with an activation energy of 0.45 eV, making the crystal unusually electrically conductive.[5][6]
The larger sized rare earth elements (lanthanides), which are those with smaller atomic number, also form trifluorides with the LaF3 structure.[4] Some actinides do as well.
Applications
This white salt is sometimes used as the "high-index" component in multilayer optical elements such as ultraviolet dichroic and narrowband mirrors. Fluorides are among the most commonly used compounds for UV optical coatings due to their relative inertness and transparency in the far ultraviolet (FUV) (100 nm < λ < 200 nm). Multilayer reflectors and antireflection coatings are typically composed of pairs of transparent materials, one with a low index of refraction, the other with a high index. LaF3 is one of very few high-index materials in the far UV.[7] The material is also a component of multimetal fluoride glasses such as ZBLAN.[8] It is also doped with europium(II) fluoride in fluoride selective electrodes.[9]
Natural occurrence
LaF3 occurs in the nature as the extremely rare mineral fluocerite-(La).[10][11] The suffix in the name is known as the Levinson modifier and, by showing the dominant element at a particular site in the structure, is used to differentiate from similar minerals (here: fluocerite-(Ce)).[12]
References
- ↑ 1.0 1.1 1.2 Haynes, William M., ed (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.69. ISBN 1439855110.
- ↑ Zalkin, A.; Templeton, D. H. (1985). "Refinement of the trigonal crystal structure of lanthanum trifluoride with neutron diffraction data". Acta Crystallographica Section B 41 (2): 91. doi:10.1107/S0108768185001689. http://journals.iucr.org/b/issues/1985/02/00/a24381/a24381.pdf.
- ↑ 3.0 3.1 "Safety Data Sheet: Lanthanum(III) fluoride". Thermo Fisher Scientific. 19 January 2018. https://www.fishersci.com/shop/msdsproxy?productName=AC199170250&productDescription=LANTHANUM(III)-FLUORIDE%252C+25GR&catNo=AC19917-0250&vendorId=VN00032119&storeId=10652.
- ↑ 4.0 4.1 4.2 Cotton, Simon (30 January 2007). Lanthanide and Actinide Chemistry. Wiley. pp. 25–27. ISBN 978-0-470-01007-5. https://books.google.com/books?id=SvAbtU6XvzgC&pg=PA26.
- ↑ Frant, Martin S.; Ross, James W. (23 December 1966). "Electrode for Sensing Fluoride Ion Activity in Solution". Science 154 (3756): 1553–1555. doi:10.1126/science.154.3756.1553. PMID 5924922. Bibcode: 1966Sci...154.1553F. https://www.jstor.org/stable/pdf/1720460.pdf.
- ↑ Sher, A.; Solomon, R.; Lee, K.; Muller, M. W. (15 April 1966). "Transport Properties of La F 3". Physical Review 144 (2): 593–604. doi:10.1103/PhysRev.144.593. Bibcode: 1966PhRv..144..593S.
- ↑ Rodríguez-de Marcos, Luis (23 September 2015). Lequime, Michel; MacLeod, H. Angus; Ristau, Detlev. eds. "Multilayers and optical constants of various fluorides in the far UV". Proceedings of SPIE: Advances in Optical Thin Films V. Optical Systems Design 2015: Advances in Optical Thin Films V 9627 (B0): 96270B. doi:10.1117/12.2191309. Bibcode: 2015SPIE.9627E..0BR. http://spie.org/Publications/Proceedings/Paper/10.1117/12.2191309. Retrieved 27 February 2019.
- ↑ Harrington, James A.. "Infrared Fiber Optics". Rutgers University. http://irfibers.rutgers.edu/pdf_files/ir_fiber_review.pdf.
- ↑ Light, Truman S.; Cappuccino, Carleton C. (April 1975). "Determination of fluoride in toothpaste using an ion-selective electrode". Journal of Chemical Education 52 (4): 247–250. doi:10.1021/ed052p247. PMID 1133123. Bibcode: 1975JChEd..52..247L.
- ↑ "Fluocerite-(La)". https://www.mindat.org/min-1568.html.
- ↑ "List of Minerals". 21 March 2011. https://www.ima-mineralogy.org/Minlist.htm.
- ↑ Burke, Ernst A.J. (2008). "Tidying up mineral names: an IMA-CNMNC scheme for suffixes, hyphens and diacrital marks". Mineralogical Record 39 (2): 131–135. http://www.mineralogicalrecord.com. Retrieved 14 November 2020.
 |
 KSF
KSF
