Ligerin
Topic: Chemistry
 From HandWiki - Reading time: 2 min
From HandWiki - Reading time: 2 min
| |
| Names | |
|---|---|
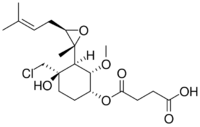
| IUPAC name
4-[(1R,2S,3S,4R)-4-(chloromethyl)-4-hydroxy-2-methoxy-3-[(2R,3R)-2-methyl-3-(3-methylbut-2-enyl)oxiran-2-yl]cyclohexyl]oxy-4-oxobutanoic acid[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C20H31ClO7 | |
| Molar mass | 418.91 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Ligerin is an antiproliferative sesquiterpene with the molecular formula C20H31ClO7 which is produced by a Penicillium species.[1][2][3]
References
- ↑ 1.0 1.1 "Ligerin" (in en). pubchem.ncbi.nlm.nih.gov. https://pubchem.ncbi.nlm.nih.gov/compound/Ligerin#section=Names-and-Identifiers.
- ↑ Barre, Stephane La; Kornprobst, Jean-Michel (5 March 2014) (in en). Outstanding Marine Molecules: Chemistry, Biology, Analysis. John Wiley & Sons. ISBN 978-3-527-68153-2.
- ↑ Vansteelandt, Marieke; Blanchet, Elodie; Egorov, Maxim; Petit, Fabien; Toupet, Loïc; Bondon, Arnaud; Monteau, Fabrice; Le Bizec, Bruno et al. (22 February 2013). "Ligerin, an Antiproliferative Chlorinated Sesquiterpenoid from a Marine-Derived Penicillium Strain". Journal of Natural Products 76 (2): 297–301. doi:10.1021/np3007364.
 |
Licensed under CC BY-SA 3.0 | Source: https://handwiki.org/wiki/Chemistry:Ligerin4 views | Status: cached on August 31 2024 23:34:42↧ Download this article as ZWI file
 KSF
KSF