List of aqueous ions by element
Topic: Chemistry
 From HandWiki - Reading time: 37 min
From HandWiki - Reading time: 37 min

Piedmontese pronunciation: [{{{1}}}]Slovak pronunciation: [{{{1}}}]text-align: auto;Expression error: Unrecognized punctuation character "{"./Expression error: Unrecognized punctuation character "{". (age Expression error: Unrecognized punctuation character "{".–Expression error: Unrecognized punctuation character "{".)Occitan pronunciation: [{{{1}}}]data-sort-value="" style="background: #ececec; color: #2C2C2C; vertical-align: middle; font-size: smaller; text-align: center; " class="table-na" | VariesHejazi pronunciation: [{{{1}}}]Spanish pronunciation: [{{{1}}}]Portuguese pronunciation: [{{{1}}}]Irish pronunciation: [{{{1}}}]Mayan pronunciation: [{{{1}}}]Nahuatl pronunciation: [{{{1}}}]Kyrgyz pronunciation: [{{{1}}}]Scottish Gaelic pronunciation: [{{{1}}}]Ukrainian pronunciation: [{{{1}}}]Burmese pronunciation: [{{{1}}}]Hindi pronunciation: [{{{1}}}]![]() Done
Done![]() To doRussian pronunciation: [{{{1}}}]Greek pronunciation: [{{{1}}}]
To doRussian pronunciation: [{{{1}}}]Greek pronunciation: [{{{1}}}]![]() Danish pronunciation: [{{{1}}}]Northern Sami pronunciation: [{{{1}}}]Mandarin pronunciation: [{{{ipa}}}]Cantonese pronunciation: [{{{1}}}]Entry from List of aqueous ions by element from TCI Europe, retrieved on {{{Date}}}
Danish pronunciation: [{{{1}}}]Northern Sami pronunciation: [{{{1}}}]Mandarin pronunciation: [{{{ipa}}}]Cantonese pronunciation: [{{{1}}}]Entry from List of aqueous ions by element from TCI Europe, retrieved on {{{Date}}}
 | This template does not display in the mobile view of Wikipedia; it is desktop only. See Template:Navbox visibility for a brief explanation. |
This is a navigational template created using {{navbox}}. It can be transcluded on pages by placing {{List of aqueous ions by element}} below the standard article appendices.
Initial visibility
This template's initial visibility currently defaults to autocollapse, meaning that if there is another collapsible item on the page (a navbox, sidebar, or table with the collapsible attribute), it is hidden apart from its title bar; if not, it is fully visible.
To change this template's initial visibility, the |state= parameter may be used:
{{List of aqueous ions by element|state=collapsed}}will show the template collapsed, i.e. hidden apart from its title bar.{{List of aqueous ions by element|state=expanded}}will show the template expanded, i.e. fully visible.
Templates using the classes class=navbox ({{navbox}}) or class=nomobile ({{sidebar}}) are not displayed in article space on the mobile web site of English Wikipedia. Mobile page views accounted for 60% to 70% of all page views from 2020 through 2025. Briefly, these templates are not included in articles because 1) they are not well designed for mobile, and 2) they significantly increase page sizes—bad for mobile downloads—in a way that is not useful for the mobile use case. You can review/watch phab:T124168 for further discussion.
TemplateData
A navigational box that can be placed at the bottom of articles.
| Parameter | Description | Type | Status | |
|---|---|---|---|---|
| State | state | The initial visibility of the navbox
| String | suggested |
Chambers, Robert; Thomson, Thomas Napier (1857). "[[s:A biographical dictionary of eminent Scotsmen/|]]". A Biographical Dictionary of Eminent Scotsmen. Glasgow: Blackie and Son.{{{1}}}Polish pronunciation: [{{{1}}}]Malagasy pronunciation: [{{{1}}}]Japanese pronunciation: [{{{1}}}]Armenian pronunciation: [{{{1}}}]Czech pronunciation: [{{{1}}}]∶Manx pronunciation: [{{{1}}}]http://www.iucnredlist.org/apps/redlist/details/full/{{{1}}}/0 Alemannic German pronunciation: [{{{1}}}]Tagalog pronunciation: [{{{1}}}]Egyptian Arabic pronunciation: [{{{1}}}]French pronunciation: [{{{1}}}]Error: Invalid time.![]() In progressPalomares, M. L. D. and Pauly, D., eds. (2011). "{{{1}}} {{{2}}}" in SeaLifeBase. April 2011 version.Turkish pronunciation: [{{{1}}}]Finnish pronunciation: [{{{1}}}]Froese, Rainer and Pauly, Daniel, eds. (2006). "{{{1}}} {{{2}}}" in FishBase. April 2006 version.
In progressPalomares, M. L. D. and Pauly, D., eds. (2011). "{{{1}}} {{{2}}}" in SeaLifeBase. April 2011 version.Turkish pronunciation: [{{{1}}}]Finnish pronunciation: [{{{1}}}]Froese, Rainer and Pauly, Daniel, eds. (2006). "{{{1}}} {{{2}}}" in FishBase. April 2006 version.
|- | ... | — | — | — | — | — | —
|-Old Norse pronunciation: [{{{1}}}]Salish pronunciation: [{{{1}}}]Basque pronunciation: [{{{1}}}]{{{1}}}data-sort-value="" style="background: #ececec; color: #2C2C2C; vertical-align: middle; font-size: smaller; text-align: center; " class="table-na" | UnreleasedNorwegian pronunciation: [{{{1}}}] (aged {{{4}}})Hungarian pronunciation: [{{{1}}}]Quechua pronunciation: [{{{1}}}]Arabic pronunciation: [{{{1}}}]Punjabi pronunciation: [{{{1}}}]Afrikaans pronunciation: [{{{1}}}]Romanian pronunciation: [{{{1}}}]Hebrew pronunciation: [{{{1}}}][INVALID OR MISSING PARAMETER IN TEMPLATE List of aqueous ions by element]Uto-Aztecan pronunciation: [{{{1}}}]Tamil pronunciation: [{{{1}}}]Hindustani pronunciation: [{{{1}}}]Swedish pronunciation: [{{{1}}}]Kazakh pronunciation: [{{{1}}}]
Lao pronunciation: [{{{1}}}]Tibetan pronunciation: [{{{1}}}]Khmer pronunciation: [{{{1}}}]data-sort-value="" style="vertical-align:middle; text-align:center" class="table-na" | —{{{1}}}Welsh pronunciation: [{{{1}}}]![]() Partially doneAthabaskan pronunciation: [{{{1}}}]Māori pronunciation: [{{{1}}}]IPA: [{{{1}}}]Bulgarian pronunciation: [{{{1}}}]Korean pronunciation: [{{{1}}}]Icelandic pronunciation: [{{{1}}}]Sanskrit pronunciation: [{{{1}}}]Bengali pronunciation: [{{{1}}}]Indonesian pronunciation: [{{{1}}}]Serbo-Croatian pronunciation: [{{{1}}}]CROSBI {{{1}}} the red cobalt cation Co2+ from Co(NO
Partially doneAthabaskan pronunciation: [{{{1}}}]Māori pronunciation: [{{{1}}}]IPA: [{{{1}}}]Bulgarian pronunciation: [{{{1}}}]Korean pronunciation: [{{{1}}}]Icelandic pronunciation: [{{{1}}}]Sanskrit pronunciation: [{{{1}}}]Bengali pronunciation: [{{{1}}}]Indonesian pronunciation: [{{{1}}}]Serbo-Croatian pronunciation: [{{{1}}}]CROSBI {{{1}}} the red cobalt cation Co2+ from Co(NO
3)
2 (see § Co)
This page may be too long to read and navigate comfortably. |
Slovene pronunciation: [{{{1}}}]Thai pronunciation: [{{{1}}}]Neukirch, Jürgen; Schmidt, Alexander; Wingberg, Kay (2000), Cohomology of Number Fields, Grundlehren der Mathematischen Wissenschaften, 323, Berlin: Springer-Verlag, ISBN 978-3-540-66671-4Template:ScribuntoPersian pronunciation: [{{{1}}}]{| class="wikitable" width="100%"
! rowspan="3" colspan="2" width="14%" style="border-bottom:2px solid grey;" | Date/Time (UTC) ! Configuration ! Serial number ! Launch site ! Outcome |-
|style="text-align:center;background-color:#e3e9e9;" | Payload |style="text-align:center;background-color:#e3e9e9;" | Separation orbit |style="text-align:center;background-color:#e3e9e9;" | Operator |style="text-align:center;background-color:#e3e9e9;" | Function |- | colspan="4" style="text-align:center;background-color:#e4dfdf;border-bottom:2px solid grey;" | Remarks |-
Parameter 1=time required!
 | This template does not display in the mobile view of Wikipedia; it is desktop only. See Template:Navbox visibility for a brief explanation. |
This is a navigational template created using {{navbox}}. It can be transcluded on pages by placing {{List of aqueous ions by element}} below the standard article appendices.
Initial visibility
This template's initial visibility currently defaults to autocollapse, meaning that if there is another collapsible item on the page (a navbox, sidebar, or table with the collapsible attribute), it is hidden apart from its title bar; if not, it is fully visible.
To change this template's initial visibility, the |state= parameter may be used:
{{List of aqueous ions by element|state=collapsed}}will show the template collapsed, i.e. hidden apart from its title bar.{{List of aqueous ions by element|state=expanded}}will show the template expanded, i.e. fully visible.
Templates using the classes class=navbox ({{navbox}}) or class=nomobile ({{sidebar}}) are not displayed in article space on the mobile web site of English Wikipedia. Mobile page views accounted for 60% to 70% of all page views from 2020 through 2025. Briefly, these templates are not included in articles because 1) they are not well designed for mobile, and 2) they significantly increase page sizes—bad for mobile downloads—in a way that is not useful for the mobile use case. You can review/watch phab:T124168 for further discussion.
TemplateData
A navigational box that can be placed at the bottom of articles.
| Parameter | Description | Type | Status | |
|---|---|---|---|---|
| State | state | The initial visibility of the navbox
| String | suggested |
Template transclusions
| Transclusion maintenance |
|---|
| Check completeness of transclusions |
Hawaiian pronunciation: [{{{1}}}]German pronunciation: [{{{1}}}]Dutch pronunciation: [{{{1}}}]Belarusian pronunciation: [{{{1}}}]Catalan pronunciation: [{{{1}}}]{{{1}}}Mongolian pronunciation: [{{{1}}}]Javanese pronunciation: [{{{1}}}]![]() Not doneLatin pronunciation: [{{{1}}}]
Not doneLatin pronunciation: [{{{1}}}]
| rowspan="1" colspan="2" style="border-top:2px solid #aabbcc;" |
| style="border-top:2px solid #aabbcc;" | | style="border-top:2px solid #aabbcc;" | | style="border-top:2px solid #aabbcc;" | | style="border-top:2px solid #aabbcc;" |
|-
|-
Vietnamese pronunciation: [{{{1}}}]
Piedmontese pronunciation: [{{{1}}}]Slovak pronunciation: [{{{1}}}]text-align: auto;Expression error: Unrecognized punctuation character "{"./Expression error: Unrecognized punctuation character "{". (age Expression error: Unrecognized punctuation character "{".–Expression error: Unrecognized punctuation character "{".)Occitan pronunciation: [{{{1}}}]data-sort-value="" style="background: #ececec; color: #2C2C2C; vertical-align: middle; font-size: smaller; text-align: center; " class="table-na" | VariesHejazi pronunciation: [{{{1}}}]Spanish pronunciation: [{{{1}}}]Portuguese pronunciation: [{{{1}}}]Irish pronunciation: [{{{1}}}]Mayan pronunciation: [{{{1}}}]Nahuatl pronunciation: [{{{1}}}]Kyrgyz pronunciation: [{{{1}}}]Scottish Gaelic pronunciation: [{{{1}}}]Ukrainian pronunciation: [{{{1}}}]Burmese pronunciation: [{{{1}}}]Hindi pronunciation: [{{{1}}}]![]() Done
Done![]() To doRussian pronunciation: [{{{1}}}]Greek pronunciation: [{{{1}}}]
To doRussian pronunciation: [{{{1}}}]Greek pronunciation: [{{{1}}}]![]() Danish pronunciation: [{{{1}}}]Northern Sami pronunciation: [{{{1}}}]Mandarin pronunciation: [{{{ipa}}}]Cantonese pronunciation: [{{{1}}}]Entry from List of aqueous ions by element from TCI Europe, retrieved on {{{Date}}}
Danish pronunciation: [{{{1}}}]Northern Sami pronunciation: [{{{1}}}]Mandarin pronunciation: [{{{ipa}}}]Cantonese pronunciation: [{{{1}}}]Entry from List of aqueous ions by element from TCI Europe, retrieved on {{{Date}}}
 | This template does not display in the mobile view of Wikipedia; it is desktop only. See Template:Navbox visibility for a brief explanation. |
This is a navigational template created using {{navbox}}. It can be transcluded on pages by placing {{List of aqueous ions by element}} below the standard article appendices.
Initial visibility
This template's initial visibility currently defaults to autocollapse, meaning that if there is another collapsible item on the page (a navbox, sidebar, or table with the collapsible attribute), it is hidden apart from its title bar; if not, it is fully visible.
To change this template's initial visibility, the |state= parameter may be used:
{{List of aqueous ions by element|state=collapsed}}will show the template collapsed, i.e. hidden apart from its title bar.{{List of aqueous ions by element|state=expanded}}will show the template expanded, i.e. fully visible.
Templates using the classes class=navbox ({{navbox}}) or class=nomobile ({{sidebar}}) are not displayed in article space on the mobile web site of English Wikipedia. Mobile page views accounted for 60% to 70% of all page views from 2020 through 2025. Briefly, these templates are not included in articles because 1) they are not well designed for mobile, and 2) they significantly increase page sizes—bad for mobile downloads—in a way that is not useful for the mobile use case. You can review/watch phab:T124168 for further discussion.
TemplateData
A navigational box that can be placed at the bottom of articles.
| Parameter | Description | Type | Status | |
|---|---|---|---|---|
| State | state | The initial visibility of the navbox
| String | suggested |
Chambers, Robert; Thomson, Thomas Napier (1857). "[[s:A biographical dictionary of eminent Scotsmen/|]]". A Biographical Dictionary of Eminent Scotsmen. Glasgow: Blackie and Son.{{{1}}}Polish pronunciation: [{{{1}}}]Malagasy pronunciation: [{{{1}}}]Japanese pronunciation: [{{{1}}}]Armenian pronunciation: [{{{1}}}]Czech pronunciation: [{{{1}}}]∶Manx pronunciation: [{{{1}}}]http://www.iucnredlist.org/apps/redlist/details/full/{{{1}}}/0 Alemannic German pronunciation: [{{{1}}}]Tagalog pronunciation: [{{{1}}}]Egyptian Arabic pronunciation: [{{{1}}}]French pronunciation: [{{{1}}}]Error: Invalid time.![]() In progressPalomares, M. L. D. and Pauly, D., eds. (2011). "{{{1}}} {{{2}}}" in SeaLifeBase. April 2011 version.Turkish pronunciation: [{{{1}}}]Finnish pronunciation: [{{{1}}}]Froese, Rainer and Pauly, Daniel, eds. (2006). "{{{1}}} {{{2}}}" in FishBase. April 2006 version.
In progressPalomares, M. L. D. and Pauly, D., eds. (2011). "{{{1}}} {{{2}}}" in SeaLifeBase. April 2011 version.Turkish pronunciation: [{{{1}}}]Finnish pronunciation: [{{{1}}}]Froese, Rainer and Pauly, Daniel, eds. (2006). "{{{1}}} {{{2}}}" in FishBase. April 2006 version.
|- | ... | — | — | — | — | — | —
|-Old Norse pronunciation: [{{{1}}}]Salish pronunciation: [{{{1}}}]Basque pronunciation: [{{{1}}}]{{{1}}}data-sort-value="" style="background: #ececec; color: #2C2C2C; vertical-align: middle; font-size: smaller; text-align: center; " class="table-na" | UnreleasedNorwegian pronunciation: [{{{1}}}] (aged {{{4}}})Hungarian pronunciation: [{{{1}}}]Quechua pronunciation: [{{{1}}}]Arabic pronunciation: [{{{1}}}]Punjabi pronunciation: [{{{1}}}]Afrikaans pronunciation: [{{{1}}}]Romanian pronunciation: [{{{1}}}]Hebrew pronunciation: [{{{1}}}][INVALID OR MISSING PARAMETER IN TEMPLATE List of aqueous ions by element]Uto-Aztecan pronunciation: [{{{1}}}]Tamil pronunciation: [{{{1}}}]Hindustani pronunciation: [{{{1}}}]Swedish pronunciation: [{{{1}}}]Kazakh pronunciation: [{{{1}}}]
Lao pronunciation: [{{{1}}}]Tibetan pronunciation: [{{{1}}}]Khmer pronunciation: [{{{1}}}]data-sort-value="" style="vertical-align:middle; text-align:center" class="table-na" | —{{{1}}}Welsh pronunciation: [{{{1}}}]![]() Partially doneAthabaskan pronunciation: [{{{1}}}]Māori pronunciation: [{{{1}}}]IPA: [{{{1}}}]Bulgarian pronunciation: [{{{1}}}]Korean pronunciation: [{{{1}}}]Icelandic pronunciation: [{{{1}}}]Sanskrit pronunciation: [{{{1}}}]Bengali pronunciation: [{{{1}}}]Indonesian pronunciation: [{{{1}}}]Serbo-Croatian pronunciation: [{{{1}}}]CROSBI {{{1}}} the orange chromium oxyanion Cr
Partially doneAthabaskan pronunciation: [{{{1}}}]Māori pronunciation: [{{{1}}}]IPA: [{{{1}}}]Bulgarian pronunciation: [{{{1}}}]Korean pronunciation: [{{{1}}}]Icelandic pronunciation: [{{{1}}}]Sanskrit pronunciation: [{{{1}}}]Bengali pronunciation: [{{{1}}}]Indonesian pronunciation: [{{{1}}}]Serbo-Croatian pronunciation: [{{{1}}}]CROSBI {{{1}}} the orange chromium oxyanion Cr
2O2−
7 from K
2Cr
2O
7 (§ Cr)
This page may be too long to read and navigate comfortably. |
Slovene pronunciation: [{{{1}}}]Thai pronunciation: [{{{1}}}]Neukirch, Jürgen; Schmidt, Alexander; Wingberg, Kay (2000), Cohomology of Number Fields, Grundlehren der Mathematischen Wissenschaften, 323, Berlin: Springer-Verlag, ISBN 978-3-540-66671-4Template:ScribuntoPersian pronunciation: [{{{1}}}]{| class="wikitable" width="100%"
! rowspan="3" colspan="2" width="14%" style="border-bottom:2px solid grey;" | Date/Time (UTC) ! Configuration ! Serial number ! Launch site ! Outcome |-
|style="text-align:center;background-color:#e3e9e9;" | Payload |style="text-align:center;background-color:#e3e9e9;" | Separation orbit |style="text-align:center;background-color:#e3e9e9;" | Operator |style="text-align:center;background-color:#e3e9e9;" | Function |- | colspan="4" style="text-align:center;background-color:#e4dfdf;border-bottom:2px solid grey;" | Remarks |-
Parameter 1=time required!
 | This template does not display in the mobile view of Wikipedia; it is desktop only. See Template:Navbox visibility for a brief explanation. |
This is a navigational template created using {{navbox}}. It can be transcluded on pages by placing {{List of aqueous ions by element}} below the standard article appendices.
Initial visibility
This template's initial visibility currently defaults to autocollapse, meaning that if there is another collapsible item on the page (a navbox, sidebar, or table with the collapsible attribute), it is hidden apart from its title bar; if not, it is fully visible.
To change this template's initial visibility, the |state= parameter may be used:
{{List of aqueous ions by element|state=collapsed}}will show the template collapsed, i.e. hidden apart from its title bar.{{List of aqueous ions by element|state=expanded}}will show the template expanded, i.e. fully visible.
Templates using the classes class=navbox ({{navbox}}) or class=nomobile ({{sidebar}}) are not displayed in article space on the mobile web site of English Wikipedia. Mobile page views accounted for 60% to 70% of all page views from 2020 through 2025. Briefly, these templates are not included in articles because 1) they are not well designed for mobile, and 2) they significantly increase page sizes—bad for mobile downloads—in a way that is not useful for the mobile use case. You can review/watch phab:T124168 for further discussion.
TemplateData
A navigational box that can be placed at the bottom of articles.
| Parameter | Description | Type | Status | |
|---|---|---|---|---|
| State | state | The initial visibility of the navbox
| String | suggested |
Template transclusions
| Transclusion maintenance |
|---|
| Check completeness of transclusions |
Hawaiian pronunciation: [{{{1}}}]German pronunciation: [{{{1}}}]Dutch pronunciation: [{{{1}}}]Belarusian pronunciation: [{{{1}}}]Catalan pronunciation: [{{{1}}}]{{{1}}}Mongolian pronunciation: [{{{1}}}]Javanese pronunciation: [{{{1}}}]![]() Not doneLatin pronunciation: [{{{1}}}]
Not doneLatin pronunciation: [{{{1}}}]
| rowspan="1" colspan="2" style="border-top:2px solid #aabbcc;" |
| style="border-top:2px solid #aabbcc;" | | style="border-top:2px solid #aabbcc;" | | style="border-top:2px solid #aabbcc;" | | style="border-top:2px solid #aabbcc;" |
|-
|-
Vietnamese pronunciation: [{{{1}}}]
Piedmontese pronunciation: [{{{1}}}]Slovak pronunciation: [{{{1}}}]text-align: auto;Expression error: Unrecognized punctuation character "{"./Expression error: Unrecognized punctuation character "{". (age Expression error: Unrecognized punctuation character "{".–Expression error: Unrecognized punctuation character "{".)Occitan pronunciation: [{{{1}}}]data-sort-value="" style="background: #ececec; color: #2C2C2C; vertical-align: middle; font-size: smaller; text-align: center; " class="table-na" | VariesHejazi pronunciation: [{{{1}}}]Spanish pronunciation: [{{{1}}}]Portuguese pronunciation: [{{{1}}}]Irish pronunciation: [{{{1}}}]Mayan pronunciation: [{{{1}}}]Nahuatl pronunciation: [{{{1}}}]Kyrgyz pronunciation: [{{{1}}}]Scottish Gaelic pronunciation: [{{{1}}}]Ukrainian pronunciation: [{{{1}}}]Burmese pronunciation: [{{{1}}}]Hindi pronunciation: [{{{1}}}]![]() Done
Done![]() To doRussian pronunciation: [{{{1}}}]Greek pronunciation: [{{{1}}}]
To doRussian pronunciation: [{{{1}}}]Greek pronunciation: [{{{1}}}]![]() Danish pronunciation: [{{{1}}}]Northern Sami pronunciation: [{{{1}}}]Mandarin pronunciation: [{{{ipa}}}]Cantonese pronunciation: [{{{1}}}]Entry from List of aqueous ions by element from TCI Europe, retrieved on {{{Date}}}
Danish pronunciation: [{{{1}}}]Northern Sami pronunciation: [{{{1}}}]Mandarin pronunciation: [{{{ipa}}}]Cantonese pronunciation: [{{{1}}}]Entry from List of aqueous ions by element from TCI Europe, retrieved on {{{Date}}}
 | This template does not display in the mobile view of Wikipedia; it is desktop only. See Template:Navbox visibility for a brief explanation. |
This is a navigational template created using {{navbox}}. It can be transcluded on pages by placing {{List of aqueous ions by element}} below the standard article appendices.
Initial visibility
This template's initial visibility currently defaults to autocollapse, meaning that if there is another collapsible item on the page (a navbox, sidebar, or table with the collapsible attribute), it is hidden apart from its title bar; if not, it is fully visible.
To change this template's initial visibility, the |state= parameter may be used:
{{List of aqueous ions by element|state=collapsed}}will show the template collapsed, i.e. hidden apart from its title bar.{{List of aqueous ions by element|state=expanded}}will show the template expanded, i.e. fully visible.
Templates using the classes class=navbox ({{navbox}}) or class=nomobile ({{sidebar}}) are not displayed in article space on the mobile web site of English Wikipedia. Mobile page views accounted for 60% to 70% of all page views from 2020 through 2025. Briefly, these templates are not included in articles because 1) they are not well designed for mobile, and 2) they significantly increase page sizes—bad for mobile downloads—in a way that is not useful for the mobile use case. You can review/watch phab:T124168 for further discussion.
TemplateData
A navigational box that can be placed at the bottom of articles.
| Parameter | Description | Type | Status | |
|---|---|---|---|---|
| State | state | The initial visibility of the navbox
| String | suggested |
Chambers, Robert; Thomson, Thomas Napier (1857). "[[s:A biographical dictionary of eminent Scotsmen/|]]". A Biographical Dictionary of Eminent Scotsmen. Glasgow: Blackie and Son.{{{1}}}Polish pronunciation: [{{{1}}}]Malagasy pronunciation: [{{{1}}}]Japanese pronunciation: [{{{1}}}]Armenian pronunciation: [{{{1}}}]Czech pronunciation: [{{{1}}}]∶Manx pronunciation: [{{{1}}}]http://www.iucnredlist.org/apps/redlist/details/full/{{{1}}}/0 Alemannic German pronunciation: [{{{1}}}]Tagalog pronunciation: [{{{1}}}]Egyptian Arabic pronunciation: [{{{1}}}]French pronunciation: [{{{1}}}]Error: Invalid time.![]() In progressPalomares, M. L. D. and Pauly, D., eds. (2011). "{{{1}}} {{{2}}}" in SeaLifeBase. April 2011 version.Turkish pronunciation: [{{{1}}}]Finnish pronunciation: [{{{1}}}]Froese, Rainer and Pauly, Daniel, eds. (2006). "{{{1}}} {{{2}}}" in FishBase. April 2006 version.
In progressPalomares, M. L. D. and Pauly, D., eds. (2011). "{{{1}}} {{{2}}}" in SeaLifeBase. April 2011 version.Turkish pronunciation: [{{{1}}}]Finnish pronunciation: [{{{1}}}]Froese, Rainer and Pauly, Daniel, eds. (2006). "{{{1}}} {{{2}}}" in FishBase. April 2006 version.
|- | ... | — | — | — | — | — | —
|-Old Norse pronunciation: [{{{1}}}]Salish pronunciation: [{{{1}}}]Basque pronunciation: [{{{1}}}]{{{1}}}data-sort-value="" style="background: #ececec; color: #2C2C2C; vertical-align: middle; font-size: smaller; text-align: center; " class="table-na" | UnreleasedNorwegian pronunciation: [{{{1}}}] (aged {{{4}}})Hungarian pronunciation: [{{{1}}}]Quechua pronunciation: [{{{1}}}]Arabic pronunciation: [{{{1}}}]Punjabi pronunciation: [{{{1}}}]Afrikaans pronunciation: [{{{1}}}]Romanian pronunciation: [{{{1}}}]Hebrew pronunciation: [{{{1}}}][INVALID OR MISSING PARAMETER IN TEMPLATE List of aqueous ions by element]Uto-Aztecan pronunciation: [{{{1}}}]Tamil pronunciation: [{{{1}}}]Hindustani pronunciation: [{{{1}}}]Swedish pronunciation: [{{{1}}}]Kazakh pronunciation: [{{{1}}}]
Lao pronunciation: [{{{1}}}]Tibetan pronunciation: [{{{1}}}]Khmer pronunciation: [{{{1}}}]data-sort-value="" style="vertical-align:middle; text-align:center" class="table-na" | —{{{1}}}Welsh pronunciation: [{{{1}}}]![]() Partially doneAthabaskan pronunciation: [{{{1}}}]Māori pronunciation: [{{{1}}}]IPA: [{{{1}}}]Bulgarian pronunciation: [{{{1}}}]Korean pronunciation: [{{{1}}}]Icelandic pronunciation: [{{{1}}}]Sanskrit pronunciation: [{{{1}}}]Bengali pronunciation: [{{{1}}}]Indonesian pronunciation: [{{{1}}}]Serbo-Croatian pronunciation: [{{{1}}}]CROSBI {{{1}}} the yellow chromium oxyanion CrO2−
Partially doneAthabaskan pronunciation: [{{{1}}}]Māori pronunciation: [{{{1}}}]IPA: [{{{1}}}]Bulgarian pronunciation: [{{{1}}}]Korean pronunciation: [{{{1}}}]Icelandic pronunciation: [{{{1}}}]Sanskrit pronunciation: [{{{1}}}]Bengali pronunciation: [{{{1}}}]Indonesian pronunciation: [{{{1}}}]Serbo-Croatian pronunciation: [{{{1}}}]CROSBI {{{1}}} the yellow chromium oxyanion CrO2−
4 from K
2CrO
4 (§ Cr)
This page may be too long to read and navigate comfortably. |
Slovene pronunciation: [{{{1}}}]Thai pronunciation: [{{{1}}}]Neukirch, Jürgen; Schmidt, Alexander; Wingberg, Kay (2000), Cohomology of Number Fields, Grundlehren der Mathematischen Wissenschaften, 323, Berlin: Springer-Verlag, ISBN 978-3-540-66671-4Template:ScribuntoPersian pronunciation: [{{{1}}}]{| class="wikitable" width="100%"
! rowspan="3" colspan="2" width="14%" style="border-bottom:2px solid grey;" | Date/Time (UTC) ! Configuration ! Serial number ! Launch site ! Outcome |-
|style="text-align:center;background-color:#e3e9e9;" | Payload |style="text-align:center;background-color:#e3e9e9;" | Separation orbit |style="text-align:center;background-color:#e3e9e9;" | Operator |style="text-align:center;background-color:#e3e9e9;" | Function |- | colspan="4" style="text-align:center;background-color:#e4dfdf;border-bottom:2px solid grey;" | Remarks |-
Parameter 1=time required!
 | This template does not display in the mobile view of Wikipedia; it is desktop only. See Template:Navbox visibility for a brief explanation. |
This is a navigational template created using {{navbox}}. It can be transcluded on pages by placing {{List of aqueous ions by element}} below the standard article appendices.
Initial visibility
This template's initial visibility currently defaults to autocollapse, meaning that if there is another collapsible item on the page (a navbox, sidebar, or table with the collapsible attribute), it is hidden apart from its title bar; if not, it is fully visible.
To change this template's initial visibility, the |state= parameter may be used:
{{List of aqueous ions by element|state=collapsed}}will show the template collapsed, i.e. hidden apart from its title bar.{{List of aqueous ions by element|state=expanded}}will show the template expanded, i.e. fully visible.
Templates using the classes class=navbox ({{navbox}}) or class=nomobile ({{sidebar}}) are not displayed in article space on the mobile web site of English Wikipedia. Mobile page views accounted for 60% to 70% of all page views from 2020 through 2025. Briefly, these templates are not included in articles because 1) they are not well designed for mobile, and 2) they significantly increase page sizes—bad for mobile downloads—in a way that is not useful for the mobile use case. You can review/watch phab:T124168 for further discussion.
TemplateData
A navigational box that can be placed at the bottom of articles.
| Parameter | Description | Type | Status | |
|---|---|---|---|---|
| State | state | The initial visibility of the navbox
| String | suggested |
Template transclusions
| Transclusion maintenance |
|---|
| Check completeness of transclusions |
Hawaiian pronunciation: [{{{1}}}]German pronunciation: [{{{1}}}]Dutch pronunciation: [{{{1}}}]Belarusian pronunciation: [{{{1}}}]Catalan pronunciation: [{{{1}}}]{{{1}}}Mongolian pronunciation: [{{{1}}}]Javanese pronunciation: [{{{1}}}]![]() Not doneLatin pronunciation: [{{{1}}}]
Not doneLatin pronunciation: [{{{1}}}]
| rowspan="1" colspan="2" style="border-top:2px solid #aabbcc;" |
| style="border-top:2px solid #aabbcc;" | | style="border-top:2px solid #aabbcc;" | | style="border-top:2px solid #aabbcc;" | | style="border-top:2px solid #aabbcc;" |
|-
|-
Vietnamese pronunciation: [{{{1}}}]
Piedmontese pronunciation: [{{{1}}}]Slovak pronunciation: [{{{1}}}]text-align: auto;Expression error: Unrecognized punctuation character "{"./Expression error: Unrecognized punctuation character "{". (age Expression error: Unrecognized punctuation character "{".–Expression error: Unrecognized punctuation character "{".)Occitan pronunciation: [{{{1}}}]data-sort-value="" style="background: #ececec; color: #2C2C2C; vertical-align: middle; font-size: smaller; text-align: center; " class="table-na" | VariesHejazi pronunciation: [{{{1}}}]Spanish pronunciation: [{{{1}}}]Portuguese pronunciation: [{{{1}}}]Irish pronunciation: [{{{1}}}]Mayan pronunciation: [{{{1}}}]Nahuatl pronunciation: [{{{1}}}]Kyrgyz pronunciation: [{{{1}}}]Scottish Gaelic pronunciation: [{{{1}}}]Ukrainian pronunciation: [{{{1}}}]Burmese pronunciation: [{{{1}}}]Hindi pronunciation: [{{{1}}}]![]() Done
Done![]() To doRussian pronunciation: [{{{1}}}]Greek pronunciation: [{{{1}}}]
To doRussian pronunciation: [{{{1}}}]Greek pronunciation: [{{{1}}}]![]() Danish pronunciation: [{{{1}}}]Northern Sami pronunciation: [{{{1}}}]Mandarin pronunciation: [{{{ipa}}}]Cantonese pronunciation: [{{{1}}}]Entry from List of aqueous ions by element from TCI Europe, retrieved on {{{Date}}}
Danish pronunciation: [{{{1}}}]Northern Sami pronunciation: [{{{1}}}]Mandarin pronunciation: [{{{ipa}}}]Cantonese pronunciation: [{{{1}}}]Entry from List of aqueous ions by element from TCI Europe, retrieved on {{{Date}}}
 | This template does not display in the mobile view of Wikipedia; it is desktop only. See Template:Navbox visibility for a brief explanation. |
This is a navigational template created using {{navbox}}. It can be transcluded on pages by placing {{List of aqueous ions by element}} below the standard article appendices.
Initial visibility
This template's initial visibility currently defaults to autocollapse, meaning that if there is another collapsible item on the page (a navbox, sidebar, or table with the collapsible attribute), it is hidden apart from its title bar; if not, it is fully visible.
To change this template's initial visibility, the |state= parameter may be used:
{{List of aqueous ions by element|state=collapsed}}will show the template collapsed, i.e. hidden apart from its title bar.{{List of aqueous ions by element|state=expanded}}will show the template expanded, i.e. fully visible.
Templates using the classes class=navbox ({{navbox}}) or class=nomobile ({{sidebar}}) are not displayed in article space on the mobile web site of English Wikipedia. Mobile page views accounted for 60% to 70% of all page views from 2020 through 2025. Briefly, these templates are not included in articles because 1) they are not well designed for mobile, and 2) they significantly increase page sizes—bad for mobile downloads—in a way that is not useful for the mobile use case. You can review/watch phab:T124168 for further discussion.
TemplateData
A navigational box that can be placed at the bottom of articles.
| Parameter | Description | Type | Status | |
|---|---|---|---|---|
| State | state | The initial visibility of the navbox
| String | suggested |
Chambers, Robert; Thomson, Thomas Napier (1857). "[[s:A biographical dictionary of eminent Scotsmen/|]]". A Biographical Dictionary of Eminent Scotsmen. Glasgow: Blackie and Son.{{{1}}}Polish pronunciation: [{{{1}}}]Malagasy pronunciation: [{{{1}}}]Japanese pronunciation: [{{{1}}}]Armenian pronunciation: [{{{1}}}]Czech pronunciation: [{{{1}}}]∶Manx pronunciation: [{{{1}}}]http://www.iucnredlist.org/apps/redlist/details/full/{{{1}}}/0 Alemannic German pronunciation: [{{{1}}}]Tagalog pronunciation: [{{{1}}}]Egyptian Arabic pronunciation: [{{{1}}}]French pronunciation: [{{{1}}}]Error: Invalid time.![]() In progressPalomares, M. L. D. and Pauly, D., eds. (2011). "{{{1}}} {{{2}}}" in SeaLifeBase. April 2011 version.Turkish pronunciation: [{{{1}}}]Finnish pronunciation: [{{{1}}}]Froese, Rainer and Pauly, Daniel, eds. (2006). "{{{1}}} {{{2}}}" in FishBase. April 2006 version.
In progressPalomares, M. L. D. and Pauly, D., eds. (2011). "{{{1}}} {{{2}}}" in SeaLifeBase. April 2011 version.Turkish pronunciation: [{{{1}}}]Finnish pronunciation: [{{{1}}}]Froese, Rainer and Pauly, Daniel, eds. (2006). "{{{1}}} {{{2}}}" in FishBase. April 2006 version.
|- | ... | — | — | — | — | — | —
|-Old Norse pronunciation: [{{{1}}}]Salish pronunciation: [{{{1}}}]Basque pronunciation: [{{{1}}}]{{{1}}}data-sort-value="" style="background: #ececec; color: #2C2C2C; vertical-align: middle; font-size: smaller; text-align: center; " class="table-na" | UnreleasedNorwegian pronunciation: [{{{1}}}] (aged {{{4}}})Hungarian pronunciation: [{{{1}}}]Quechua pronunciation: [{{{1}}}]Arabic pronunciation: [{{{1}}}]Punjabi pronunciation: [{{{1}}}]Afrikaans pronunciation: [{{{1}}}]Romanian pronunciation: [{{{1}}}]Hebrew pronunciation: [{{{1}}}][INVALID OR MISSING PARAMETER IN TEMPLATE List of aqueous ions by element]Uto-Aztecan pronunciation: [{{{1}}}]Tamil pronunciation: [{{{1}}}]Hindustani pronunciation: [{{{1}}}]Swedish pronunciation: [{{{1}}}]Kazakh pronunciation: [{{{1}}}]
Lao pronunciation: [{{{1}}}]Tibetan pronunciation: [{{{1}}}]Khmer pronunciation: [{{{1}}}]data-sort-value="" style="vertical-align:middle; text-align:center" class="table-na" | —{{{1}}}Welsh pronunciation: [{{{1}}}]![]() Partially doneAthabaskan pronunciation: [{{{1}}}]Māori pronunciation: [{{{1}}}]IPA: [{{{1}}}]Bulgarian pronunciation: [{{{1}}}]Korean pronunciation: [{{{1}}}]Icelandic pronunciation: [{{{1}}}]Sanskrit pronunciation: [{{{1}}}]Bengali pronunciation: [{{{1}}}]Indonesian pronunciation: [{{{1}}}]Serbo-Croatian pronunciation: [{{{1}}}]CROSBI {{{1}}} the turquoise nickel cation Ni2+ from NiCl
Partially doneAthabaskan pronunciation: [{{{1}}}]Māori pronunciation: [{{{1}}}]IPA: [{{{1}}}]Bulgarian pronunciation: [{{{1}}}]Korean pronunciation: [{{{1}}}]Icelandic pronunciation: [{{{1}}}]Sanskrit pronunciation: [{{{1}}}]Bengali pronunciation: [{{{1}}}]Indonesian pronunciation: [{{{1}}}]Serbo-Croatian pronunciation: [{{{1}}}]CROSBI {{{1}}} the turquoise nickel cation Ni2+ from NiCl
2 (§ Ni)
This page may be too long to read and navigate comfortably. |
Slovene pronunciation: [{{{1}}}]Thai pronunciation: [{{{1}}}]Neukirch, Jürgen; Schmidt, Alexander; Wingberg, Kay (2000), Cohomology of Number Fields, Grundlehren der Mathematischen Wissenschaften, 323, Berlin: Springer-Verlag, ISBN 978-3-540-66671-4Template:ScribuntoPersian pronunciation: [{{{1}}}]{| class="wikitable" width="100%"
! rowspan="3" colspan="2" width="14%" style="border-bottom:2px solid grey;" | Date/Time (UTC) ! Configuration ! Serial number ! Launch site ! Outcome |-
|style="text-align:center;background-color:#e3e9e9;" | Payload |style="text-align:center;background-color:#e3e9e9;" | Separation orbit |style="text-align:center;background-color:#e3e9e9;" | Operator |style="text-align:center;background-color:#e3e9e9;" | Function |- | colspan="4" style="text-align:center;background-color:#e4dfdf;border-bottom:2px solid grey;" | Remarks |-
Parameter 1=time required!
 | This template does not display in the mobile view of Wikipedia; it is desktop only. See Template:Navbox visibility for a brief explanation. |
This is a navigational template created using {{navbox}}. It can be transcluded on pages by placing {{List of aqueous ions by element}} below the standard article appendices.
Initial visibility
This template's initial visibility currently defaults to autocollapse, meaning that if there is another collapsible item on the page (a navbox, sidebar, or table with the collapsible attribute), it is hidden apart from its title bar; if not, it is fully visible.
To change this template's initial visibility, the |state= parameter may be used:
{{List of aqueous ions by element|state=collapsed}}will show the template collapsed, i.e. hidden apart from its title bar.{{List of aqueous ions by element|state=expanded}}will show the template expanded, i.e. fully visible.
Templates using the classes class=navbox ({{navbox}}) or class=nomobile ({{sidebar}}) are not displayed in article space on the mobile web site of English Wikipedia. Mobile page views accounted for 60% to 70% of all page views from 2020 through 2025. Briefly, these templates are not included in articles because 1) they are not well designed for mobile, and 2) they significantly increase page sizes—bad for mobile downloads—in a way that is not useful for the mobile use case. You can review/watch phab:T124168 for further discussion.
TemplateData
A navigational box that can be placed at the bottom of articles.
| Parameter | Description | Type | Status | |
|---|---|---|---|---|
| State | state | The initial visibility of the navbox
| String | suggested |
Template transclusions
| Transclusion maintenance |
|---|
| Check completeness of transclusions |
Hawaiian pronunciation: [{{{1}}}]German pronunciation: [{{{1}}}]Dutch pronunciation: [{{{1}}}]Belarusian pronunciation: [{{{1}}}]Catalan pronunciation: [{{{1}}}]{{{1}}}Mongolian pronunciation: [{{{1}}}]Javanese pronunciation: [{{{1}}}]![]() Not doneLatin pronunciation: [{{{1}}}]
Not doneLatin pronunciation: [{{{1}}}]
| rowspan="1" colspan="2" style="border-top:2px solid #aabbcc;" |
| style="border-top:2px solid #aabbcc;" | | style="border-top:2px solid #aabbcc;" | | style="border-top:2px solid #aabbcc;" | | style="border-top:2px solid #aabbcc;" |
|-
|-
Vietnamese pronunciation: [{{{1}}}]
Piedmontese pronunciation: [{{{1}}}]Slovak pronunciation: [{{{1}}}]text-align: auto;Expression error: Unrecognized punctuation character "{"./Expression error: Unrecognized punctuation character "{". (age Expression error: Unrecognized punctuation character "{".–Expression error: Unrecognized punctuation character "{".)Occitan pronunciation: [{{{1}}}]data-sort-value="" style="background: #ececec; color: #2C2C2C; vertical-align: middle; font-size: smaller; text-align: center; " class="table-na" | VariesHejazi pronunciation: [{{{1}}}]Spanish pronunciation: [{{{1}}}]Portuguese pronunciation: [{{{1}}}]Irish pronunciation: [{{{1}}}]Mayan pronunciation: [{{{1}}}]Nahuatl pronunciation: [{{{1}}}]Kyrgyz pronunciation: [{{{1}}}]Scottish Gaelic pronunciation: [{{{1}}}]Ukrainian pronunciation: [{{{1}}}]Burmese pronunciation: [{{{1}}}]Hindi pronunciation: [{{{1}}}]![]() Done
Done![]() To doRussian pronunciation: [{{{1}}}]Greek pronunciation: [{{{1}}}]
To doRussian pronunciation: [{{{1}}}]Greek pronunciation: [{{{1}}}]![]() Danish pronunciation: [{{{1}}}]Northern Sami pronunciation: [{{{1}}}]Mandarin pronunciation: [{{{ipa}}}]Cantonese pronunciation: [{{{1}}}]Entry from List of aqueous ions by element from TCI Europe, retrieved on {{{Date}}}
Danish pronunciation: [{{{1}}}]Northern Sami pronunciation: [{{{1}}}]Mandarin pronunciation: [{{{ipa}}}]Cantonese pronunciation: [{{{1}}}]Entry from List of aqueous ions by element from TCI Europe, retrieved on {{{Date}}}
 | This template does not display in the mobile view of Wikipedia; it is desktop only. See Template:Navbox visibility for a brief explanation. |
This is a navigational template created using {{navbox}}. It can be transcluded on pages by placing {{List of aqueous ions by element}} below the standard article appendices.
Initial visibility
This template's initial visibility currently defaults to autocollapse, meaning that if there is another collapsible item on the page (a navbox, sidebar, or table with the collapsible attribute), it is hidden apart from its title bar; if not, it is fully visible.
To change this template's initial visibility, the |state= parameter may be used:
{{List of aqueous ions by element|state=collapsed}}will show the template collapsed, i.e. hidden apart from its title bar.{{List of aqueous ions by element|state=expanded}}will show the template expanded, i.e. fully visible.
Templates using the classes class=navbox ({{navbox}}) or class=nomobile ({{sidebar}}) are not displayed in article space on the mobile web site of English Wikipedia. Mobile page views accounted for 60% to 70% of all page views from 2020 through 2025. Briefly, these templates are not included in articles because 1) they are not well designed for mobile, and 2) they significantly increase page sizes—bad for mobile downloads—in a way that is not useful for the mobile use case. You can review/watch phab:T124168 for further discussion.
TemplateData
A navigational box that can be placed at the bottom of articles.
| Parameter | Description | Type | Status | |
|---|---|---|---|---|
| State | state | The initial visibility of the navbox
| String | suggested |
Chambers, Robert; Thomson, Thomas Napier (1857). "[[s:A biographical dictionary of eminent Scotsmen/|]]". A Biographical Dictionary of Eminent Scotsmen. Glasgow: Blackie and Son.{{{1}}}Polish pronunciation: [{{{1}}}]Malagasy pronunciation: [{{{1}}}]Japanese pronunciation: [{{{1}}}]Armenian pronunciation: [{{{1}}}]Czech pronunciation: [{{{1}}}]∶Manx pronunciation: [{{{1}}}]http://www.iucnredlist.org/apps/redlist/details/full/{{{1}}}/0 Alemannic German pronunciation: [{{{1}}}]Tagalog pronunciation: [{{{1}}}]Egyptian Arabic pronunciation: [{{{1}}}]French pronunciation: [{{{1}}}]Error: Invalid time.![]() In progressPalomares, M. L. D. and Pauly, D., eds. (2011). "{{{1}}} {{{2}}}" in SeaLifeBase. April 2011 version.Turkish pronunciation: [{{{1}}}]Finnish pronunciation: [{{{1}}}]Froese, Rainer and Pauly, Daniel, eds. (2006). "{{{1}}} {{{2}}}" in FishBase. April 2006 version.
In progressPalomares, M. L. D. and Pauly, D., eds. (2011). "{{{1}}} {{{2}}}" in SeaLifeBase. April 2011 version.Turkish pronunciation: [{{{1}}}]Finnish pronunciation: [{{{1}}}]Froese, Rainer and Pauly, Daniel, eds. (2006). "{{{1}}} {{{2}}}" in FishBase. April 2006 version.
|- | ... | — | — | — | — | — | —
|-Old Norse pronunciation: [{{{1}}}]Salish pronunciation: [{{{1}}}]Basque pronunciation: [{{{1}}}]{{{1}}}data-sort-value="" style="background: #ececec; color: #2C2C2C; vertical-align: middle; font-size: smaller; text-align: center; " class="table-na" | UnreleasedNorwegian pronunciation: [{{{1}}}] (aged {{{4}}})Hungarian pronunciation: [{{{1}}}]Quechua pronunciation: [{{{1}}}]Arabic pronunciation: [{{{1}}}]Punjabi pronunciation: [{{{1}}}]Afrikaans pronunciation: [{{{1}}}]Romanian pronunciation: [{{{1}}}]Hebrew pronunciation: [{{{1}}}][INVALID OR MISSING PARAMETER IN TEMPLATE List of aqueous ions by element]Uto-Aztecan pronunciation: [{{{1}}}]Tamil pronunciation: [{{{1}}}]Hindustani pronunciation: [{{{1}}}]Swedish pronunciation: [{{{1}}}]Kazakh pronunciation: [{{{1}}}]
Lao pronunciation: [{{{1}}}]Tibetan pronunciation: [{{{1}}}]Khmer pronunciation: [{{{1}}}]data-sort-value="" style="vertical-align:middle; text-align:center" class="table-na" | —{{{1}}}Welsh pronunciation: [{{{1}}}]![]() Partially doneAthabaskan pronunciation: [{{{1}}}]Māori pronunciation: [{{{1}}}]IPA: [{{{1}}}]Bulgarian pronunciation: [{{{1}}}]Korean pronunciation: [{{{1}}}]Icelandic pronunciation: [{{{1}}}]Sanskrit pronunciation: [{{{1}}}]Bengali pronunciation: [{{{1}}}]Indonesian pronunciation: [{{{1}}}]Serbo-Croatian pronunciation: [{{{1}}}]CROSBI {{{1}}} the blue copper cation Cu2+ from CuSO
Partially doneAthabaskan pronunciation: [{{{1}}}]Māori pronunciation: [{{{1}}}]IPA: [{{{1}}}]Bulgarian pronunciation: [{{{1}}}]Korean pronunciation: [{{{1}}}]Icelandic pronunciation: [{{{1}}}]Sanskrit pronunciation: [{{{1}}}]Bengali pronunciation: [{{{1}}}]Indonesian pronunciation: [{{{1}}}]Serbo-Croatian pronunciation: [{{{1}}}]CROSBI {{{1}}} the blue copper cation Cu2+ from CuSO
4 (§ Cu)
This page may be too long to read and navigate comfortably. |
Slovene pronunciation: [{{{1}}}]Thai pronunciation: [{{{1}}}]Neukirch, Jürgen; Schmidt, Alexander; Wingberg, Kay (2000), Cohomology of Number Fields, Grundlehren der Mathematischen Wissenschaften, 323, Berlin: Springer-Verlag, ISBN 978-3-540-66671-4Template:ScribuntoPersian pronunciation: [{{{1}}}]{| class="wikitable" width="100%"
! rowspan="3" colspan="2" width="14%" style="border-bottom:2px solid grey;" | Date/Time (UTC) ! Configuration ! Serial number ! Launch site ! Outcome |-
|style="text-align:center;background-color:#e3e9e9;" | Payload |style="text-align:center;background-color:#e3e9e9;" | Separation orbit |style="text-align:center;background-color:#e3e9e9;" | Operator |style="text-align:center;background-color:#e3e9e9;" | Function |- | colspan="4" style="text-align:center;background-color:#e4dfdf;border-bottom:2px solid grey;" | Remarks |-
Parameter 1=time required!
 | This template does not display in the mobile view of Wikipedia; it is desktop only. See Template:Navbox visibility for a brief explanation. |
This is a navigational template created using {{navbox}}. It can be transcluded on pages by placing {{List of aqueous ions by element}} below the standard article appendices.
Initial visibility
This template's initial visibility currently defaults to autocollapse, meaning that if there is another collapsible item on the page (a navbox, sidebar, or table with the collapsible attribute), it is hidden apart from its title bar; if not, it is fully visible.
To change this template's initial visibility, the |state= parameter may be used:
{{List of aqueous ions by element|state=collapsed}}will show the template collapsed, i.e. hidden apart from its title bar.{{List of aqueous ions by element|state=expanded}}will show the template expanded, i.e. fully visible.
Templates using the classes class=navbox ({{navbox}}) or class=nomobile ({{sidebar}}) are not displayed in article space on the mobile web site of English Wikipedia. Mobile page views accounted for 60% to 70% of all page views from 2020 through 2025. Briefly, these templates are not included in articles because 1) they are not well designed for mobile, and 2) they significantly increase page sizes—bad for mobile downloads—in a way that is not useful for the mobile use case. You can review/watch phab:T124168 for further discussion.
TemplateData
A navigational box that can be placed at the bottom of articles.
| Parameter | Description | Type | Status | |
|---|---|---|---|---|
| State | state | The initial visibility of the navbox
| String | suggested |
Template transclusions
| Transclusion maintenance |
|---|
| Check completeness of transclusions |
Hawaiian pronunciation: [{{{1}}}]German pronunciation: [{{{1}}}]Dutch pronunciation: [{{{1}}}]Belarusian pronunciation: [{{{1}}}]Catalan pronunciation: [{{{1}}}]{{{1}}}Mongolian pronunciation: [{{{1}}}]Javanese pronunciation: [{{{1}}}]![]() Not doneLatin pronunciation: [{{{1}}}]
Not doneLatin pronunciation: [{{{1}}}]
| rowspan="1" colspan="2" style="border-top:2px solid #aabbcc;" |
| style="border-top:2px solid #aabbcc;" | | style="border-top:2px solid #aabbcc;" | | style="border-top:2px solid #aabbcc;" | | style="border-top:2px solid #aabbcc;" |
|-
|-
Vietnamese pronunciation: [{{{1}}}]
Piedmontese pronunciation: [{{{1}}}]Slovak pronunciation: [{{{1}}}]text-align: auto;Expression error: Unrecognized punctuation character "{"./Expression error: Unrecognized punctuation character "{". (age Expression error: Unrecognized punctuation character "{".–Expression error: Unrecognized punctuation character "{".)Occitan pronunciation: [{{{1}}}]data-sort-value="" style="background: #ececec; color: #2C2C2C; vertical-align: middle; font-size: smaller; text-align: center; " class="table-na" | VariesHejazi pronunciation: [{{{1}}}]Spanish pronunciation: [{{{1}}}]Portuguese pronunciation: [{{{1}}}]Irish pronunciation: [{{{1}}}]Mayan pronunciation: [{{{1}}}]Nahuatl pronunciation: [{{{1}}}]Kyrgyz pronunciation: [{{{1}}}]Scottish Gaelic pronunciation: [{{{1}}}]Ukrainian pronunciation: [{{{1}}}]Burmese pronunciation: [{{{1}}}]Hindi pronunciation: [{{{1}}}]![]() Done
Done![]() To doRussian pronunciation: [{{{1}}}]Greek pronunciation: [{{{1}}}]
To doRussian pronunciation: [{{{1}}}]Greek pronunciation: [{{{1}}}]![]() Danish pronunciation: [{{{1}}}]Northern Sami pronunciation: [{{{1}}}]Mandarin pronunciation: [{{{ipa}}}]Cantonese pronunciation: [{{{1}}}]Entry from List of aqueous ions by element from TCI Europe, retrieved on {{{Date}}}
Danish pronunciation: [{{{1}}}]Northern Sami pronunciation: [{{{1}}}]Mandarin pronunciation: [{{{ipa}}}]Cantonese pronunciation: [{{{1}}}]Entry from List of aqueous ions by element from TCI Europe, retrieved on {{{Date}}}
 | This template does not display in the mobile view of Wikipedia; it is desktop only. See Template:Navbox visibility for a brief explanation. |
This is a navigational template created using {{navbox}}. It can be transcluded on pages by placing {{List of aqueous ions by element}} below the standard article appendices.
Initial visibility
This template's initial visibility currently defaults to autocollapse, meaning that if there is another collapsible item on the page (a navbox, sidebar, or table with the collapsible attribute), it is hidden apart from its title bar; if not, it is fully visible.
To change this template's initial visibility, the |state= parameter may be used:
{{List of aqueous ions by element|state=collapsed}}will show the template collapsed, i.e. hidden apart from its title bar.{{List of aqueous ions by element|state=expanded}}will show the template expanded, i.e. fully visible.
Templates using the classes class=navbox ({{navbox}}) or class=nomobile ({{sidebar}}) are not displayed in article space on the mobile web site of English Wikipedia. Mobile page views accounted for 60% to 70% of all page views from 2020 through 2025. Briefly, these templates are not included in articles because 1) they are not well designed for mobile, and 2) they significantly increase page sizes—bad for mobile downloads—in a way that is not useful for the mobile use case. You can review/watch phab:T124168 for further discussion.
TemplateData
A navigational box that can be placed at the bottom of articles.
| Parameter | Description | Type | Status | |
|---|---|---|---|---|
| State | state | The initial visibility of the navbox
| String | suggested |
Chambers, Robert; Thomson, Thomas Napier (1857). "[[s:A biographical dictionary of eminent Scotsmen/|]]". A Biographical Dictionary of Eminent Scotsmen. Glasgow: Blackie and Son.{{{1}}}Polish pronunciation: [{{{1}}}]Malagasy pronunciation: [{{{1}}}]Japanese pronunciation: [{{{1}}}]Armenian pronunciation: [{{{1}}}]Czech pronunciation: [{{{1}}}]∶Manx pronunciation: [{{{1}}}]http://www.iucnredlist.org/apps/redlist/details/full/{{{1}}}/0 Alemannic German pronunciation: [{{{1}}}]Tagalog pronunciation: [{{{1}}}]Egyptian Arabic pronunciation: [{{{1}}}]French pronunciation: [{{{1}}}]Error: Invalid time.![]() In progressPalomares, M. L. D. and Pauly, D., eds. (2011). "{{{1}}} {{{2}}}" in SeaLifeBase. April 2011 version.Turkish pronunciation: [{{{1}}}]Finnish pronunciation: [{{{1}}}]Froese, Rainer and Pauly, Daniel, eds. (2006). "{{{1}}} {{{2}}}" in FishBase. April 2006 version.
In progressPalomares, M. L. D. and Pauly, D., eds. (2011). "{{{1}}} {{{2}}}" in SeaLifeBase. April 2011 version.Turkish pronunciation: [{{{1}}}]Finnish pronunciation: [{{{1}}}]Froese, Rainer and Pauly, Daniel, eds. (2006). "{{{1}}} {{{2}}}" in FishBase. April 2006 version.
|- | ... | — | — | — | — | — | —
|-Old Norse pronunciation: [{{{1}}}]Salish pronunciation: [{{{1}}}]Basque pronunciation: [{{{1}}}]{{{1}}}data-sort-value="" style="background: #ececec; color: #2C2C2C; vertical-align: middle; font-size: smaller; text-align: center; " class="table-na" | UnreleasedNorwegian pronunciation: [{{{1}}}] (aged {{{4}}})Hungarian pronunciation: [{{{1}}}]Quechua pronunciation: [{{{1}}}]Arabic pronunciation: [{{{1}}}]Punjabi pronunciation: [{{{1}}}]Afrikaans pronunciation: [{{{1}}}]Romanian pronunciation: [{{{1}}}]Hebrew pronunciation: [{{{1}}}][INVALID OR MISSING PARAMETER IN TEMPLATE List of aqueous ions by element]Uto-Aztecan pronunciation: [{{{1}}}]Tamil pronunciation: [{{{1}}}]Hindustani pronunciation: [{{{1}}}]Swedish pronunciation: [{{{1}}}]Kazakh pronunciation: [{{{1}}}]
Lao pronunciation: [{{{1}}}]Tibetan pronunciation: [{{{1}}}]Khmer pronunciation: [{{{1}}}]data-sort-value="" style="vertical-align:middle; text-align:center" class="table-na" | —{{{1}}}Welsh pronunciation: [{{{1}}}]![]() Partially doneAthabaskan pronunciation: [{{{1}}}]Māori pronunciation: [{{{1}}}]IPA: [{{{1}}}]Bulgarian pronunciation: [{{{1}}}]Korean pronunciation: [{{{1}}}]Icelandic pronunciation: [{{{1}}}]Sanskrit pronunciation: [{{{1}}}]Bengali pronunciation: [{{{1}}}]Indonesian pronunciation: [{{{1}}}]Serbo-Croatian pronunciation: [{{{1}}}]CROSBI {{{1}}} the purple manganese oxyanion MnO−
Partially doneAthabaskan pronunciation: [{{{1}}}]Māori pronunciation: [{{{1}}}]IPA: [{{{1}}}]Bulgarian pronunciation: [{{{1}}}]Korean pronunciation: [{{{1}}}]Icelandic pronunciation: [{{{1}}}]Sanskrit pronunciation: [{{{1}}}]Bengali pronunciation: [{{{1}}}]Indonesian pronunciation: [{{{1}}}]Serbo-Croatian pronunciation: [{{{1}}}]CROSBI {{{1}}} the purple manganese oxyanion MnO−
4 from KMnO
4 (§ Mn)]]
This table lists the ionic species that are most likely to be present, depending on pH, in aqueous solutions of binary salts of metal ions. The existence must be inferred on the basis of indirect evidence provided by modelling with experimental data or by analogy with structures obtained by X-ray crystallography.
Introduction
When a salt of a metal ion, with the generic formula MXn, is dissolved in water, it will dissociate into a cation and anions.[citation needed]
(aq) signifies that the ion is aquated, with cations having a chemical formula [M(H2O)p]q+ and anions whose state of aquation is generally unknown. For convenience (aq) is not shown in the rest of this article as the number of water molecules that are attached to the ions is irrelevant in regard to hydrolysis. This reaction occurs quantitatively with salts of the alkali-metals at low to moderate concentrations.[citation needed]
With salts of divalent metal ions, the aqua-ion will be subject to a dissociation reaction, known as hydrolysis, a name derived from Greek words for water splitting. The first step in this process can be written as[citation needed]
- ⇌
When the pH of the solution is increased by adding an alkaline solution to it, the extent of hydrolysis increases. Measurements of pH or colour change are used to derive the equilibrium constant for the reaction. Further hydrolysis may occur, producing dimeric, trimeric or polymeric species containing hydroxy- or oxy- groups. The next step is to determine which model for the chemical processes best fits the experimental data.[citation needed]
Model selection
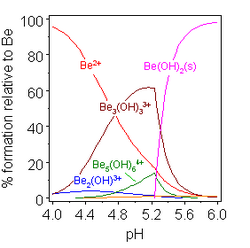
The model is defined in terms of a list of those complex species which are present in solutions in significant amounts. In the present context the complex species have the general formula [MpOq(OH)r]n±. where p, q and r define the stoichiometry of the species and n± gives the electrical charge of the ion. The experimental data are fitted to those models which may represent the species that are formed in solution. The model which gives the best fit is selected for publication. However, the pH range in which data may be collected is limited by the fact that an hydroxide with formula M(OH)n will be formed at relatively low pH, as illustrated at the right. This will make the process of model selection difficult when monomers and dimers are formed. and virtually impossible when higher polymers are also formed. In those cases it must be assumed that the species found in solids are also present in solutions.
The formation of an hydroxo-bridged species is enthalpically favoured over the monomers, countering the unfavourable entropic effect of aggregation. For this reason, it is difficult to establish models in which both types of species are present.
Monomeric hydrolysis products
The extent of hydrolysis can be quantified when the values of the hydrolysis constants can be determined experimentally. The first hydrolysis constant refers to the equilibrium
- ⇌
The association constant for this reaction can be expressed as
- (electrical charges are omitted from generic expressions)
Numerical values for this equilibrium constant can be found in papers concerned only with metal ion hydrolysis. However, it is more useful, in general, to use the acid dissociation constant, Ka.
and to cite the cologarithm, pKa, of the value of this quantity in books and other publications. The two values are constrained by the relationship
- log K(association) * log K(dissociation) = pKw
pKw refers to the self-ionization of water: pK = log (1/K) = -log(K).
Further monomeric complexes may be formed in a stepwise manner.
- ⇌
Dimeric species
Hydrolysed species containing two metal ions, with the general formula M2(OH)n, may be formed from pre-existing monomeric species. The stepwise reaction
- ⇌
illustrates the process. An alternative stepwise reaction
- ⇌
may also occur. Unfortunately it is not possible to distinguish between these two possibilities using data from potentiometric titrations because both of these reactions have no effect on the pH of the solution.
The concentration of a dimeric species decreases more rapidly with metal ion concentration than does the concentration of the corresponding monomeric species. Therefore, when determining the stability constants of both species it is usually necessary to obtain data from 2 or more titrations, each with a different metal salt concentration. Otherwise the stability constant non-linear least squares refinement may fail without providing the desired values, due to there being 100% mathematical correlation between the refinement parameters for the monomeric and dimeric species.
Trimeric and polymeric species
The principal problem when determining the stability constant for a polymeric species is how to select the "best" model to use from a number of possibilities. An example that illustrates the problem is shown in Baes & Mesmer, p. 119.[1]
A trimeric species must be formed from a chemical reaction of a dimer with a monomer, with the implication that the value of the stability constant of the dimer must be "known", having been determined using separate experimental data. In practice this extremely difficult to achieve. Instead, it is generally assumed that the species in solution are the same as the species that have been identified in crystal structure determinations. There is no way to establish whether or not the assumption is justified. Furthermore, species that are required as intermediaries between the monomer and the polymer may have such low concentrations as to be "undetectable".
An extreme example concerns the species with a cluster of 13 aluminium(III) ions, which can be isolated in the solid state; there must be at least 12 intermediate species in solution, which have not been characterized. It follows that the published stoichiometry of the polymeric species in solution may well be correct, but it is always possible that other species are actually present in solution. In general, the omission of intermediary species will affect the reliability of the published speciation schemes.
Soluble hydroxides
Some hydroxides of non-metallic elements are soluble in water; they are not included in the following table. Examples cited by Baes and Mesmer (p. 413) include hydroxides of Gallium(III), Indium(III), Thallium(III), Arsenic(III), Antimony(III) and Bismuth(III). Most hydroxides of transition metals are classified as being "insoluble" in water. Some of them dissolve, with reaction, in alkaline solution.
- M(OH)n + OH− → [M(OH){n+1}]−
List
For some highly radioactive elements, such as astatine and radon, only tracer quantities have been experimented on. As such, unambiguous characterisation of the species they form is impossible, and so their species have been excluded from the table below. Some theoretical speculations as to what they might be are present in the literature; more information can be found at the main articles of the elements involved.
| Z | Element | Oxidation state |
Cations & Anions |
Oxycations & hydroxycations |
Oxyanions & hydroxyanions |
|---|---|---|---|---|---|
| 1 | Hydrogen | +1 | H+ |
||
| 2 | Helium | ||||
| 3 | Lithium | +1 | Li+ |
||
| 4 | Beryllium | +2 | Be2+ | Be(OH)+ , Be 2(OH)3+, Be 3(OH)3+ 3 |
Be(OH)− 3, Be(OH)2− 4 |
| 5 | Boron | +3 | borates | ||
| 6 | Carbon | +4 | carbonate | ||
| 7 | Nitrogen | −3 +3 +5 |
NH2-, NH4+ |
nitrite nitrate | |
| 8 | Oxygen | −2 | hydroxide | ||
| 9 | Fluorine | −1 | fluoride | ||
| 10 | Neon | ||||
| 11 | Sodium | +1 | Na+ |
||
| 12 | Magnesium | +2 | Mg2+ | Mg(OH)+ , Mg 4(OH)4+ 4 |
|
| 13 | Aluminium | +3 | Al3+ | Al(OH)2+, Al(OH)+ 2, Al 2(OH)4+ 2, Al 3(OH)5+ 4 |
aluminates |
| 14 | Silicon | +4 | silicates | ||
| 15 | Phosphorus | −2 +3 +5 |
phosphide |
P(H)O2− 3, phosphites PO3− 4, polyphosphates | |
| 16 | Sulfur | −2 +4 +6 |
HS− [2] |
sulfite sulfate | |
| 17 | Chlorine | −1 +1 +3 +5 +7 |
chloride |
hypochlorite chlorite chlorate perchlorate | |
| 18 | Argon | ||||
| 19 | Potassium | +1 | K+ |
||
| 20 | Calcium | +2 | Ca2+ | Ca(OH)+ |
|
| 21 | Scandium | +3 | Sc3+ | Sc(OH)2+, Sc(OH)+ 2, Sc 2(OH)4+ 2, Sc 3(OH)4+ 5 |
Sc(OH)− 4 |
| 22 | Titanium | +3 +4 |
Ti3+ (violet) |
Ti(OH)2+, Ti 2(OH)4+ 2 Ti(OH)+ 3 |
titanates |
| 23 | Vanadium | +2 +3 +4 +5 |
V2+ (violet) V3+ (green) |
V(OH)2+, V(OH)+ 2 (blue) VO2+, VO(OH)+ VO+ 2 (yellow) |
VO 2(OH)− 2, VO3− 4, vanadates |
| 24 | Chromium | +2 +3 +6 |
Cr2+ (blue-green) Cr3+ (green) |
Cr(OH)2+, Cr(OH)+ 2, Cr 2(OH)4+ 2, Cr 3(OH)5+ 4 |
Cr(OH)3− 6 chromate and dichromate |
| 25 | Manganese | +2 +3 +6 +7 |
Mn2+ (faint pink) |
Mn(OH)+ , Mn 2(OH)3+ Mn(OH)2+ |
manganate permanganate |
| 26 | Iron | +2 +3 +6 |
Fe2+ (green) Fe3+ (violet) |
Fe(OH)+ Fe(OH)2+, Fe(OH)+ 2, Fe 2(OH)4+ 2, Fe 3(OH)5+ 4 |
Fe(OH)− 3 Fe(OH)3− 6 FeO2− 4, ferrate(VI) |
| 27 | Cobalt | +2 +3 +5 |
Co2+ (pink) Co3+ (blue-green) |
Co(OH)+ , Co 2(OH)3+, Co 4(OH)4+ 4 |
percobaltate |
| 28 | Nickel | +2 | Ni2+ (green) | Ni(OH)+ , Ni 2(OH)3+, Ni 4(OH)4+ 4 |
oxonickelates |
| 29 | Copper | +1 +2 |
Cu+ Cu2+ (blue) |
Cu(OH)+ , Cu 2(OH)2+ 2 |
cuprates |
| 30 | Zinc | +2 | Zn2+ | Zn(OH)+ , Zn 2(OH)3+ |
Zn(OH)− 3, Zn(OH)2− 4, zincate |
| 31 | Gallium | +3 | Ga3+ | Ga(OH)2+, Ga(OH)+ 2 |
Ga(OH)− 4 |
| 32 | Germanium | +4 | GeO(OH)− 3, Ge 2(OH)2− 2, germanates | ||
| 33 | Arsenic | −3 +3 +5 |
arsenide |
As(OH)− 4, arsenite arsenate | |
| 34 | Selenium | −2 +4 +6 |
hydrogen selenide |
selenite (ion), polymeric species selenate | |
| 35 | Bromine | −1 +5 +7 |
bromide |
bromite bromate | |
| 36 | Krypton | ||||
| 37 | Rubidium | +1 | Rb+ |
||
| 38 | Strontium | +2 | Sr2+ | SrOH+ |
|
| 39 | Yttrium | +3 | Y3+ | Y(OH)2+, Y(OH)+ 2, Y 2(OH)4+ 2, Y 2(OH)5+ 3 |
Y(OH)− 4 |
| 40 | Zirconium | +4 | Zr(OH)3+, Zr 4(OH)8+ 8 |
||
| 41 | Niobium | +5 | polymeric niobates | ||
| 42 | Molybdenum | +3 +6 |
Mo3+ |
molybdate, isopolyanions | |
| 43 | Technetium | +7 | pertechnetate | ||
| 44 | Ruthenium | +2 +3 +6 +7 |
Ru2+ (pink) Ru3+ (yellow-red) |
RuO2− 4 RuO− 4 | |
| 45 | Rhodium | +3 | Rh3+ (yellow) | RhOH2+ | |
| 46 | Palladium | +2 | Pd2+ (red-brown) | PdOH+ |
|
| 47 | Silver | +1 | Ag+ |
Ag(OH)− 2 | |
| 48 | Cadmium | +2 | Cd2+ | Cd(OH)+ , Cd 2OH3+, Cd 4(OH)4+ 4 |
Cd(OH)− 3, Cd(OH)2− 4 |
| 49 | Indium | +3 | In3+ | InOH2+, In(OH)+ 2 |
In(OH)3− 6 |
| 50 | Tin | +2 +4 |
Sn2+ |
SnOH+ , Sn 2(OH)2+ 2, Sn 3(OH)2+ 4 |
Sn(OH)− 3, stannate Sn(OH)2− 6 |
| 51 | Antimony | −3 +3 +5 |
Sb3− |
Sb(OH)+ 2 |
Sb(OH)− 4 Sb(OH)− 6, antimonates |
| 52 | Tellurium | −2 +4 +6 |
HTe− , Te2− |
Te(OH)+ 3 |
TeO(OH)− 3, TeO 2(OH)3− 2 tellurate |
| 53 | Iodine | −1 +5 +7 |
iodide |
I(OH)6+ |
iodate periodate |
| 54 | Xenon | +8 | XeO4− 6 | ||
| 55 | Caesium | +1 | Cs+ |
||
| 56 | Barium | +2 | Ba2+ | Ba(OH)+ |
|
| 57 | Lanthanum | +3 | La3+ | La(OH)2+ | |
| 58 | Cerium | +3 +4 |
Ce3+ |
Ce(OH)2+ Ce(OH)2+ 2 |
|
| 59 | Praseodymium | +3 | Pr3+ (green) | Pr(OH)2+ | |
| 60 | Neodymium | +3 | Nd3+ (lilac) | Nd(OH)2+ | Nd(OH)− 4 |
| 61 | Promethium | +3 | Pm3+ (pink) | Pm(OH)2+ | |
| 62 | Samarium | +3 | Sm2+ (red) Sm3+ (yellow) |
Sm(OH)2+ | |
| 63 | Europium | +2 +3 |
Eu2+ Eu3+ (pale pink) |
Eu(OH)2+ |
|
| 64 | Gadolinium | +3 | Gd3+ | Gd(OH)2+ | Gd(OH)− 4 |
| 65 | Terbium | +3 | Tb3+ (pale pink) | Tb(OH)2+ | |
| 66 | Dysprosium | +3 | Dy3+ (yellow) | Dy(OH)2+ | Dy(OH)− 4 |
| 67 | Holmium | +3 | Ho3+ (yellow) | Ho(OH)2+ | |
| 68 | Erbium | +3 | Er3+ (yellow) | Er(OH)2+ | Er(OH)− 4 |
| 69 | Thulium | +3 | Tm3+ (pale green) | Tm(OH)2+ | |
| 70 | Ytterbium | +2 +3 |
Yb2+ (green) Yb3+ |
Yb(OH)2+ |
Yb(OH)− 4 |
| 71 | Lutetium | +3 | Lu3+ | ||
| 72 | Hafnium | +4 | Hf(OH)3+ | polymeric species | |
| 73 | Tantalum | +5 | tantalates | ||
| 74 | Tungsten | +6 | WO2− 4, tungstates | ||
| 75 | Rhenium | +7 | ReO− 4 | ||
| 76 | Osmium | +6 +8 |
[OsO 2(OH) 4]2− (purple) [OsO 4(OH) 2]2− | ||
| 77 | Iridium | +3 >+3 |
Ir(OH)2+, Ir(OH)+ 2 |
polymeric species | |
| 78 | Platinum | +2 +4 |
Pt2+ (yellow) |
polymeric species, platinum(IV) | |
| 79 | Gold | +3 | Au3+ | Au(OH)2+, Au(OH)+ 2 |
Au(OH)− 4, Au(OH)2− 5 |
| 80 | Mercury | +1 +2 |
Hg2+ 2 Hg2+ |
Hg(OH)+ , Hg 2(OH)3+ |
|
| 81 | Thallium | +1 +3 |
Tl+ |
Tl(OH)2+, Tl(OH)+ 2 |
Tl(OH)− 4 |
| 82 | Lead | +2 | Pb2+ | Pb(OH)+ , Pb 2(OH)3+, Pb 4(OH)4+ 4 |
Pb(OH)− 3 |
| 83 | Bismuth | +3 | Bi3+ | Bi(OH)2+, Bi(OH)+ 2 |
Bi(OH)− 4, polymeric species |
| 84 | Polonium | −2 +2 +4 |
polonide Po2+ |
PoO2− 3 | |
| 85 | Astatine | ||||
| 86 | Radon | ||||
| 87 | Francium | +1 | Fr+ |
||
| 88 | Radium | +2 | Ra2+ | RaOH+ |
|
| 89 | Actinium | +3 | Ac3+ | AcOH2+ | |
| 90 | Thorium | +4 | Th4+ | Th(OH)3+, Th(OH)2+ 2 |
Th 2(OH)6+ 2, polymeric species |
| 91 | Protactinium | +3 +4 +5 |
Pa3+ Pa4+ |
PaOH3+, Pa(OH)2+ 2, Pa(OH)+ 3 PaO+ 2 |
|
| 92 | Uranium | +3 +4 +6 |
U3+ (purple) U4+ (green) |
U(OH)3+ UO2+ 2, UO 2OH+ , (UO 2) 2(OH)2+ 2 |
polymeric species uranates |
| 93 | Neptunium | +3 +4 +5 +6 |
Np3+ (purple) Np4+ (green) |
Np(OH)3+ NpO+ 2 NpO2+ 2, NpO 2(OH)+ , (NpO 2) 2(OH)2+ 2 |
|
| 94 | Plutonium | +3 +4 +6 |
Pu3+ (blue lavender) Pu4+ (yellow brown) |
Pu(OH)3+ PuO 2(OH)+ , (PuO 2) 2(OH)2+ 2, (PuO 2) 3(OH)+ 5 |
|
| 95 | Americium | +3 | Am3+ (pink) | ||
| 96 | Curium | +3 | Cm3+ (yellow) | ||
| 97 | Berkelium | +3 +4 |
Bk3+ (green) Bk4+ (yellow) |
||
| 98 | Californium | +3 | Cf3+ (green) | ||
| 99 | Einsteinium | +3 | Es3+ (pale pink) | ||
| 100 | Fermium | +2 +3 |
Fm2+ Fm3+ |
||
| 101 | Mendelevium | +2 +3 |
Md2+ Md3+ |
||
| 102 | Nobelium | +2 +3 |
No2+ No3+ |
||
| 103 | Lawrencium | +3 | Lr3+ | ||
| 104 | Rf & beyond |
Lanthanide ions
| Oxidation state |
Script error: No such module "Vertical header". | Script error: No such module "Vertical header". | Script error: No such module "Vertical header". | Script error: No such module "Vertical header". | Script error: No such module "Vertical header". | Script error: No such module "Vertical header". | Script error: No such module "Vertical header". | Script error: No such module "Vertical header". | Script error: No such module "Vertical header". | Script error: No such module "Vertical header". | Script error: No such module "Vertical header". | Script error: No such module "Vertical header". | Script error: No such module "Vertical header". | Script error: No such module "Vertical header". | Script error: No such module "Vertical header". |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| +2 | Sm2+ | Eu2+ | Tm2+ | Yb2+ | |||||||||||
| +3 | La3+ | Ce3+ | Pr3+ | Nd3+ | Pm3+ | Sm3+ | Eu3+ | Gd3+ | Tb3+ | Dy3+ | Ho3+ | Er3+ | Tm3+ | Yb3+ | Lu3+ |
| +4 | Ce4+ | Pr4+ | Nd4+ | Tb4+ | Dy4+ |
Anions
| Group 15 | Group 16 | Group 17 |
|---|---|---|
| azanide | hydroxide | fluoride |
| phosphide | hydrogen sulfide[2] | chloride |
| arsenide | hydrogen selenide | bromide |
| antimonide, stibnide | hydrogen telluride | iodide |
| polonide |
Periodic table distribution
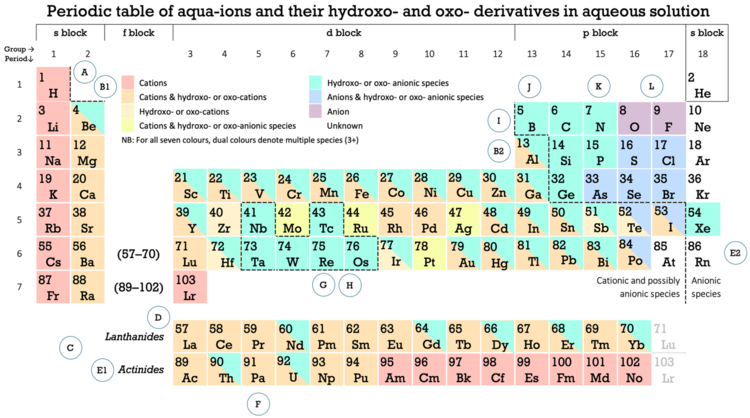
The occurrence of the different kinds of ions of the elements is shown in this periodic table:[dubious ]
Periodic table notes
Rather than the periodic table being the sum of its groups and periods[4] an examination of the image shows several patterns[5] Thus, there is a largely a left-to-right transition in metallic character seen in the red-orange-sand-yellow colours for the metals, and the turquoise, blue and violet colours for the nonmetals. The dashed line seen in the periods 1 to 4 corresponds to notions of a dividing line between metals and nonmetals. The mixed species in periods 5 and 6 shows how much trouble chemists can have in assessing where to continue the dividing line.[6][7][8] The separate dashed boundary around the Nb-Ta-W-Tc-Re-Os-Ir hexad is an exemplar for the reputation many transition metals have for nonmetallic chemistry.[9]
| Periodic table block | Positive ions | Negative ions |
|---|---|---|
| s | 93% | 7% |
| f | 88% | 12% |
| d | 49% | 51% |
| p | 32% | 68% |
| The incidence of positively charged ions (cations, oxycations and hydroxycations) and negatively charged ions (anions, oyxanions and hydroxyanions) in each block of the periodic table shows a left to right decline of positively charged ions and increase in negatively charged species, This pattern is consistent with a left to right progression in metallic to nonmetallic character.[10] | ||
Ⓐ Hydrogen is shown as being a cation former but most of its chemistry, "can be explained in terms of its tendency to [eventually] acquire the electronic configuration of…helium",[11] thereby behaving predominately as a nonmetal.
Ⓑ Beryllium has an isodiagonal relationship with aluminium, in group 13, such a relationship also occurring between B and Si; and C and P.
Ⓒ Cation-only elements are shown as being limited to sixteen elements: all those in group 1, and the heavier actinides.
Ⓓ Rare earth metals are the group 3 metals scandium, yttrium, lutetium and the lanthanides; scandium is the only such metal shown as being capable of forming an oxyanion.
Ⓔ Radioactive elements, such as the actinides, are harder to study. The known species may not represent the whole of what is possible, and the identifications may sometimes be in doubt. Astatine, as another example, is highly radioactive, and determining its stable species is "clouded by the extremely low concentrations at which astatine experiments have been conducted, and the possibility of reactions with impurities, walls and filters, or radioactivity by-products,[12] and other unwanted nano-scale interactions. Equally, as Kirby noted, “since the trace chemistry of I sometimes differs significantly from its own macroscopic chemistry, analogies drawn between At and I are likely to be questionable, at best."[13]
Ⓕ The earlier actinides, up to uranium, show some superficial resemblance to their transition metal counterparts in groups 3 to 9.[14]
Ⓖ Most of the transition metals are known for their nonmetallic chemistry, and this is particularly seen in the image for periods 5 and 6, groups 5 to 9. They nevertheless have the relatively high electrical conductivity values characteristic of metals.[15]
Ⓗ The transition metals (or d-block metals) further show electrochemical character, in terms of their capacity to form positive or negative ions, that is in-between that of (i) the s and f-block metals; and (ii) the p-block elements.[16][lower-alpha 1]
Ⓘ The p-block shows a relatively distinct cutoff in periods 1 to 4 between elements commonly recognised as metals and nonmetals. Periods 5 and 6 include elements commonly recognised as metalloids by authors who recognise such a class or subclass (antimony and tellurium), and elements less commonly recognised as such (polonium and astatine).[19]
Ⓙ Stein, in 1987,[20] showed the metalloid elements as occupying a zone in the p-block composed of B, Si, Ge, As, Sb, Po, Te, At and Rn. In the periodic table image these elements are found on the right or upper side of the dashed line traversing the p-block.
Ⓚ Of 103 elements shown in the image, just ten form anions, all of these being in the p-block: arsenic; the five chalcogens: oxygen, sulfur, selenium, tellurium, polonium; and the four halogens: fluorine, chlorine, bromine, and iodine
Ⓛ Anion-only elements are confined to oxygen and fluorine.
Further notes
- ↑ Atkins[17] discusses the transition more narrowly: Between the "virulent and violent" metals on the left of the periodic table, and the "calm and contented" metals to the right are the transition metals, which form "a transitional bridge between the two" extremes. Jensen[18] speculated that notion of "transition" elements was intended to indicate that, "these elements were undergoing a transition in the occupancy of their underlying n − 1 or n − 2 shells from 8 or 18 electrons at the beginning of the series to 18 or 32 electrons at the end of the series."
See also
Books
- Baes, CE; Mesmer, RE (1976). The Hydrolysis of Cations. Malabar: Krieger. pp. xvi+489. ISBN 0-89874-892-5.
- Brown, PW; Ekberg, C (2016). Hydrolysis of Metal Ions. Weinheim: Wiley‐VCH. pp. xvi+918. ISBN 978-3-527-33010-2.
- Richens, David T (1997). The Chemistry of Aqua Ions. New York: John Wiley & Sons. pp. xi+592. ISBN 978-0-471-97058-3.
- Turova, N (2011). Inorganic Chemistry in Tables. Berlin: Springer. pp. iv+157. ISBN 978-3-642-20487-6.
- Schweitzer, GK; Pesterfield, LL (2010). The Aqueous Chemistry of the Elements. Oxford: Oxford University Press. pp. x+448. ISBN 978-0-19-539335-4.
- Sanderson, Robert Thomas (1960). Chemical Periodicity. New York: Reinhold. pp. 330+illust.
- Greenwood, Norman, N.; Earnshaw, Alan (1984). "Chapter 2, Chemical Periodicity and the Periodic Table". Chemistry of the Elements (2nd ed.). Oxford: Butterworth. ISBN 0-7506-3365-4.
References
- ↑ Mesmer, R.E; Baes, C.F (1971). "Acidity measurements at elevated temperatures. V. Aluminum ion hydrolysis". Inorg. Chem. 1971 (10): 2290–2296. doi:10.1021/ic50104a040.
- ↑ 2.0 2.1 May, PM (2018). "Goodbye to S2− in aqueous solution". Chemical Communications 54 (16): 1980–1983. doi:10.1039/C8CC00187A. PMID 29404555.
- ↑ dtv-Atlas zur Chemie 1981, Vol. 1, p. 220.
- ↑ Bierenstiel, M; Snow, K (2019). "Periodic universe: A teaching model for understanding the periodic table of the elements". Journal of Chemical Education 96 (7): 1367–1376 (1367). doi:10.1021/acs.jchemed.8b00740. Bibcode: 2019JChEd..96.1367B.
- ↑ Rayner-Canham, G (2000). "Periodic patterns". Journal of Chemical Education 77 (8): 1053–1056. doi:10.1021/ed077p1053. Bibcode: 2000JChEd..77.1053R.
- ↑ Russell, AM; Lee, KL (2005). Structure-Property Relations in Nonferrous Metals. Hoboken: John Wiley & Sons. pp. 419, 430. ISBN 978-0-471-64952-6.; Information Sources in Metallic Materials. London: Bowker-Saur. 1989. p. 210. ISBN 978-0-408-01491-5.; Hermann, A; Hoffmann, R; Ashcroft, NW (2013). "Condensed astatine: Monatomic and metallic". Physical Review Letters 111 (11): 1604–1−11604–5. doi:10.1103/PhysRevLett.111.116404. PMID 24074111. Bibcode: 2013PhRvL.111k6404H.
- ↑ Jolly, WL (1966). The Chemistry of the Non-Metals. Englewood Cliffs, New Jersey: Prentice-Hall. pp. 64, 107.; Steudel, R (1977). Chemistry of the Non-Metals With an Introduction to Atomic Structure and Chemical Bonding. Berlin: Walter de Gruyter.; Hawkes, SJ (2010). "Polonium and astatine are not semimetals'". Journal of Chemical Education 87 (8): 783. doi:10.1021/ed100308w. Bibcode: 2010JChEd..87..783H.
- ↑ Rochow, EG (1966). The metalloids. Boston: D. C. Heath. p. 8.; Pimentel, GC; Spratley, RD (1971). Understanding chemistry. San Francisco: Holden-Day. p. 664.
- ↑ Hamm, DI (1969). Fundamental Concepts of Chemistry. New York: Appelton-Century-Crofts Press. pp. 678, 686.; Harding, C; Johnson, DA; Janes, R (2002). Elements of the p Block. Cambridge: Royal Society of Chemistry. p. 61. ISBN 978-0-85404-690-4.
- ↑ Beiser, A. Perspectives of modern physics. New York: McGraw-Hill. p. 234. "Across each period is a more or less steady transition from an active metal through less active metals and weakly active non- metals to highly active nonmetals and finally to an inert gas."
- ↑ Liptrot, GF (1983). Modern Inorganic Chemistry. London: Bell & Hyman. p. 161. ISBN 978-0-7135-1357-8.
- ↑ Viser, GWM (1989). "Inorganic astatine chemistry. Part II: The chameleon behavior and electrophilicity of At− species". Radiochimica Acta 47: 97−103 (100). doi:10.1524/ract.1989.47.23.97.
- ↑ Kirby, HW (1985). "Analytical chemistry of astatine". Gmelin Handbook of Inorganic Chemistry, At Astatine. Springer-Verlag. pp. 129−139 (129). ISBN 978-3-662-05870-1.
- ↑ Wiberg, N (1995). Inorganic Chemistry. San Diego: Academic Press. p. 1720. ISBN 978-0-12-352651-9.
- ↑ Russell, AM; Lee, KL (2005). Structure-Property Relations in Nonferrous Metals. Hoboken: John Wiley & Sons. pp. 205, 221, 243, 292. ISBN 978-0-471-64952-6.
- ↑ Raj, G (2008). Advanced Inorganic Chemistry. 1 (31 ed.). Goel. p. 1129. ISBN 978-81-87224-03-7.
- ↑ Atkins, PA (2001). The periodic kingdom: A journey into the land of the chemical elements. New York: BasicBooks. pp. 18−19. ISBN 978-0-465-07265-1.
- ↑ Jensen, WB (2003). "The place of zinc, cadmium, and mercury in the Periodic Table". Journal of Chemical Education 80 (8): 952−961 (953). doi:10.1021/ed080p952. Bibcode: 2003JChEd..80..952J.
- ↑ Vernon, RE (2013). "Which elements are metalloids". Journal of Chemical Education 90 (12): 1703–1707. doi:10.1021/ed3008457. Bibcode: 2013JChEd..90.1703V.
- ↑ Stein, L (1987). "Chemical properties of radon". in Hopke, PK. Radon and its decay products Occurrence, properties, and health effects. ACS Symposium Series. 331. Washington DS: American Chemical Society. pp. 240−251 (248). doi:10.1021/bk-1987-0331. ISBN 9780841210158.
 |
 KSF
KSF