Low valent magnesium compounds
Topic: Chemistry
 From HandWiki - Reading time: 2 min
From HandWiki - Reading time: 2 min
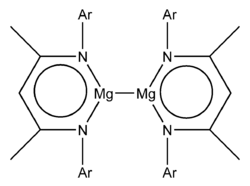
A number of stable low valent magnesium compounds containing a metal-metal, Mg-Mg, bond, where magnesium exhibits the formal oxidation state of +1 are known. These compounds generally have the formula L2Mg2, where L represents a bulky ligand.[1] The first examples of these stable magnesium(I) compounds were reported in 2007.[2] The chemistry of Mg is dominated by the +2 oxidation state and prior to 2007 only examples of crystalline compounds with short Mg-Mg distances that may indicate an Mg-Mg bond were known, such as the ternary metal hydrides Mg2RuH4, Mg3RuH3, and Mg4IrH5 and magnesium diboride,[3] Calculations had also indicated the stability of the Mg22+ cation.[4]
The preparation of the first compounds made involved the reduction of MgII iodine complexes with potassium metal and the bulky ligands were:[2]
- a guanidinate, "priso", [(Ar)NC(NPri2)N(Ar)]− where Ar = 2,6-diisopropylphenyl and Pri = iso-propyl
- a ketiminate, "nacnac", {[(Ar)NC(Me)]2CH}−,- where Ar = 2,6-diisopropylphenyl and Me = methyl
Both examples have the formula L2Mg2, where L represents the bulky anionic bidentate ligand.[2] X-ray crystallographic studies showed an Mg-Mg bond length of 285.1 pm and 284.6 pm.[2] Theoretical studies indicate an essentially ionic formulation Mg22+(L−)2.[2] The Mg22+ ion is the group 2 analogue of the group 12 Hg22+ (present in e.g. mercury(I) chloride) and Cd22+ ions (present in cadmium(I) tetrachloroaluminate).
Since then a variety of stable Mg(I) compounds have been prepared, some melting over 200 °C, some colorless, others colored, but all involving very bulky ligands.[1] Also complexes of the LMgMgL with monodentate ligands have been prepared and in these the coordination of the Mg atom increases from three to four.[1] The magnesium(I) dimers have proved to be useful reducing agents, for example in the preparation of tin(I) compounds.[5]
References
- ↑ 1.0 1.1 1.2 Stasch, Andreas; Jones, Cameron (2011). "Stable dimeric magnesium(i) compounds: from chemical landmarks to versatile reagents". Dalton Transactions 40 (21): 5659–5672. doi:10.1039/C0DT01831G. PMID 21390353.
- ↑ 2.0 2.1 2.2 2.3 2.4 Green, S. P.; Jones C.; Stasch A. (December 2007). "Stable Magnesium(I) Compounds with Mg-Mg Bonds". Science 318 (5857): 1754–1757. doi:10.1126/science.1150856. PMID 17991827. Bibcode: 2007Sci...318.1754G.
- ↑ King, R. Bruce (October 2002). "Chemical bonding topology of superconductors. 5. The similarities between magnesium diboride and cuprate superconductors and the role of subvalent magnesium". Polyhedron 21 (23): 2347–2350. doi:10.1016/S0277-5387(02)01183-X.
- ↑ Hogreve, H. (August 2004). "Mg22+: a long-lived metastable dication". Chemical Physics Letters 394 (1–3): 32–36. doi:10.1016/j.cplett.2004.06.099. Bibcode: 2004CPL...394...32H.
- ↑ Choong, Sam L.; Schenk, Christian; Stasch, Andreas; Dange, Deepak; Jones, Cameron (2012). "Contrasting reductions of group 14 metal(ii) chloride complexes: synthesis of a [small beta]-diketiminato tin(i) dimer". Chemical Communications 48 (19): 2504–2506. doi:10.1039/C2CC18086C. PMID 22281528.
 |
 KSF
KSF