MOPS
Topic: Chemistry
 From HandWiki - Reading time: 2 min
From HandWiki - Reading time: 2 min
| |
| Names | |
|---|---|
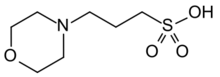
| Preferred IUPAC name
3-(Morpholin-4-yl)propane-1-sulfonic acid | |
| Other names
3-(N-Morpholino)propanesulfonic acid,
3-Morpholinopropanesulfonic acid, 3-N-Morpholino propansulfonic acid, 4-Morpholinepropanesulfonic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H15NO4S | |
| Molar mass | 209.26 g·mol−1 |
| Hazards | |
| Safety data sheet | MSDS |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
MOPS (3-(N-morpholino)propanesulfonic acid) is a buffer introduced in the 1960s, one of the twenty Good's buffers. It is a structural analog to MES,[1] and like MES, its structure contains a morpholine ring. HEPES is a similar pH buffering compound that contains a piperazine ring. With a pKa of 7.20, MOPS is an excellent buffer for many biological systems at near-neutral pH.
Applications
MOPS is frequently used as a buffering agent in biology and biochemistry. It has been tested and recommended for polyacrylamide gel electrophoresis.[2] Usage above 20 mM in mammalian cell culture work is not recommended.[3] MOPS buffer solutions become discolored (yellow) over time, but reportedly slight discoloration does not significantly affect the buffering characteristics.[4]
See also
References
- ↑ Good, Norman E.; Winget, G. Douglas; Winter, Wilhelmina; Connolly, Thomas N.; Izawa, Seikichi; Singh, Raizada M. M. (1966). "Hydrogen Ion Buffers for Biological Research". Biochemistry 5 (2): 467–77. doi:10.1021/bi00866a011. PMID 5942950.
- ↑ Thomas, J; Hodes, ME (1981). "A new discontinuous buffer system for the electrophoresis of cationic proteins at near-neutral pH". Analytical Biochemistry 118 (1): 194–6. doi:10.1016/0003-2697(81)90178-0. PMID 6278979.
- ↑ Eagle, H. (1971). "Buffer Combinations for Mammalian Cell Culture". Science 174 (4008): 500–3. doi:10.1126/science.174.4008.500. PMID 5110427. Bibcode: 1971Sci...174..500E.
- ↑ "Error: no
|title=specified when using {{Cite web}}". https://bostonbioproducts.com/products/mops-buffer-1-m-ph-74-bbm-74.
External links
- Sigma Aldrich Buffer Calculator - Useful tool to calculate weight, volume, or concentration from molecular weight.
- Recipe for MOPS buffer on OpenWetWare
 |
 KSF
KSF