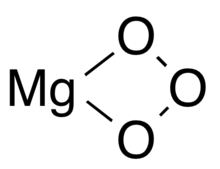
Magnesium ozonide
Topic: Chemistry
 From HandWiki - Reading time: 2 min
From HandWiki - Reading time: 2 min
| |
| Identifiers | |
|---|---|
| |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| MgO3 | |
| Molar mass | 72.302 g·mol−1 |
| Appearance | White solid |
| Related compounds | |
Other cations
|
Potassium ozonide, Ammonium ozonide |
Related compounds
|
Magnesium oxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Magnesium ozonide is a compound with the formula MgO3. Much like other ozonides, it is only stable at low temperatures.[1] Unlike other ozonide compounds, magnesium ozonide is white rather than the typical red colour.[2]
Preparation
Magnesium ozonide can be made by passing a dilute mixture of ozone in liquid nitrogen and over magnesium at -259 °C.[1]
[math]\ce{ O3 + Mg -> MgO3 }[/math]
Magnesium bisonozide
Magnesium is also known to form bisozonide complexes, containing Mg(O3)2 complexed with argon or carbon monoxide, in an argon matrix.[3]
References
- ↑ 1.0 1.1 Andrews, Lester; Prochaska, Eleanor S.; Ault, Bruce S. (1978-07-15). "Matrix reactions of magnesium atoms with ozone. Infrared spectra of MgO, MgO2, and MgO3 in solid nitrogen" (in en). The Journal of Chemical Physics 69 (2): 556–563. doi:10.1063/1.436646. ISSN 0021-9606. Bibcode: 1978JChPh..69..556A.
- ↑ Vol’nov, Il’ya Ivanovich (1966). Petrocelli, A. W.. ed (in en). Peroxides, Superoxides, and Ozonides of Alkali and Alkaline Earth Metals. Boston, MA: Springer US. doi:10.1007/978-1-4684-8252-2. ISBN 978-1-4684-8254-6. http://link.springer.com/10.1007/978-1-4684-8252-2.
- ↑ Wang, Guanjun; Gong, Yu; Zhang, Qingqing; Zhou, Mingfei (2010). "Formation and Characterization of Magnesium Bisozonide and Carbonyl Complexes in Solid Argon". Journal of Physical Chemistry A 114 (40): 10803–10809. doi:10.1021/jp107434f. PMID 20857987. Bibcode: 2010JPCA..11410803W.
 |
Licensed under CC BY-SA 3.0 | Source: https://handwiki.org/wiki/Chemistry:Magnesium_ozonide8 views | Status: cached on August 04 2024 15:48:54↧ Download this article as ZWI file
 KSF
KSF