Maleic anhydride
Topic: Chemistry
 From HandWiki - Reading time: 8 min
From HandWiki - Reading time: 8 min
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Furan-2,5-dione[2] | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 106909 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| 2728 | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2215 | ||
| |||
| |||
| Properties | |||
| C4H2O3 | |||
| Molar mass | 98.057 g·mol−1 | ||
| Appearance | White crystals or needles[3] | ||
| Odor | irritating, choking[3] | ||
| Density | 1.48 g/cm3 | ||
| Melting point | 52.8 °C (127.0 °F; 325.9 K) | ||
| Boiling point | 202 °C (396 °F; 475 K) | ||
| Reacts | |||
| Vapor pressure | 0.2 mmHg (20°C)[3] | ||
| −35.8·10−6 cm3/mol | |||
| Hazards | |||
| Safety data sheet | MSDS at J. T. Baker | ||
| GHS pictograms |   
| ||
| GHS Signal word | Danger | ||
| H302, H314, H317, H334, H372 | |||
| P260, P261, P264, P270, P272, P280, P285, P301+312, P301+330+331, P302+352, P303+361+353, P304+340, P304+341, P305+351+338, P310, P314, P321, P330, P333+313, P342+311, P363, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 102 °C (216 °F; 375 K) | ||
| Explosive limits | 1.4%-7.1%[3] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
465 mg/kg (oral, mouse) 850 mg/kg (oral, rat) 875 mg/kg (oral, rabbit) 390 mg/kg (oral, guinea pig) 400 mg/kg (oral, rat)[4] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 1 mg/m3 (0.25 ppm)[3] | ||
REL (Recommended)
|
TWA 1 mg/m3 (0.25 ppm)[3] | ||
IDLH (Immediate danger)
|
10 mg/m3[3] | ||
| Related compounds | |||
Related acid anhydrides
|
Succinic anhydride | ||
Related compounds
|
Maleic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
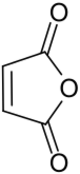
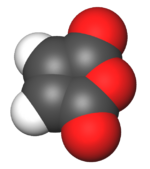
Maleic anhydride is an organic compound with the formula C
2H
2(CO)
2O. It is the acid anhydride of maleic acid. It is a colorless or white solid with an acrid odor. It is produced industrially on a large scale for applications in coatings and polymers.[5]
Structure and bonding
Maleic anhydride is a planar molecule. By virtue of the acid anhydride group, the alkene is electrophilic.
On account of its cycle of 4 π electrons in an array of 5 atoms with p orbitals, maleic anhydride was long thought to exhibit antiaromaticity. However, a thermochemical study concluded that only 8 kJ/mol of destabilization energy can be ascribed to this effect, making it weakly antiaromatic at best.[6]
Production
Maleic anhydride is produced by vapor-phase oxidation of n-butane. The overall process converts the methyl groups to carboxylate and dehydrogenates the backbone. The selectivity of the process reflects the robustness of maleic anhydride, with its conjugated double-bond system. Traditionally maleic anhydride was produced by the oxidation of benzene or other aromatic compounds. As of 2006, only a few smaller plants continue to use benzene.
In both cases, benzene and butane are fed into a stream of hot air, and the mixture is passed through a catalyst bed at high temperature. The ratio of air to hydrocarbon is controlled to prevent the mixture from igniting. Vanadium pentoxide and molybdenum trioxide are the catalysts used for the benzene route, whereas vanadium phosphate is used for the butane route:[5]
- C
4H
10 + 3.5 O
2 → C
4H
2O
3 + 4 H
2O ∆H = −1236 kJ/mol
The main competing process entails full combustion of the butane, a conversion that is twice as exothermic as the partial oxidation.
The traditional method using benzene became uneconomical due to the high and still rising benzene prices and by complying with the regulations of benzene emissions. In addition, in the production of maleic anhydride (4 C-atoms) a third of the original carbon atoms is lost as carbon dioxide when using benzene (6 carbon atoms). The modern catalytic processes start from a 4-carbon molecule and only attaches oxygen and removes water; the 4-C-base body of the molecule remains intact. Overall, the newer method is therefore more material efficient.[7]
Parallels exist with the production of phthalic anhydride: While older methods use naphthalene, modern methods use o-xylene as feedstock.
Reactions

The chemistry of maleic anhydride is very rich, reflecting its ready availability and bifunctional reactivity. Maleic anhydride hydrolyzes, producing maleic acid, cis–HOOC–CH=CH–COOH. With alcohols, the half-ester is generated, e.g., cis–HOOC–CH=CH–COOCH
3. With amines, maleic anhydride gives maleamic acids.[8]
Maleic anhydride is a classic substrate for Diels-Alder reactions.[9] It was used for work in 1928, on the reaction between maleic anhydride and 1,3-butadiene, for which Otto Paul Hermann Diels and Kurt Alder were awarded the Nobel Prize in 1950. It is through this reaction that maleic anhydride is converted to many pesticides and pharmaceuticals. Their 1928 patent also provided many other examples of reactions involving maleic anhydride, such as the reaction with cyclopentadiene to form nadic anhydride.[10]
- 450px
At higher temperatures, the half salts, half esters of maleic acid undergo the Michael reaction with active methylene or methine compounds such as malonate or acetoacetate esters in the presence of sodium acetate catalyst. These intermediates were used for the same Krebs cycle intermediates aconitic and isocitric acids.[11][12]
Maleic anhydride dimerizes in a photochemical reaction to form cyclobutane tetracarboxylic dianhydride (CBTA). This compound is used in the production of polyimides and as an alignment film for liquid crystal displays.[13]
It is also a ligand forming metal-alkene complexes, examples being Pt(PPh
3)
2(MA) and Fe(CO)
4(MA).[14]
Uses
-
Malathion is a popular insecticide derived from maleic anhydride
-
Sodium sulfosuccinate esters, common class of surfactants derived from maleic anhydride
-
Alkenylsuccinic anhydrides, which are derived from maleic anhydride, are widely used in papermaking
-
Rubratoxin A is one of many natural products containing a maleic anhydride-like subunit.
Plastics & resins
Around 50% of world maleic anhydride output is used in the manufacture of unsaturated polyester resins (UPR). These resins are used in diverse applications such as vehicles, construction, furniture, and machinery.[15] Chopped glass fibers are added to UPR to produce fiberglass reinforced plastics.
Curing agents
Diels-Alder reaction of maleic anhydride and butadiene and isoprene gives the respective tetrahydrophthalic anhydrides which can be hydrogenated to the corresponding hexahydrophthalic anhydrides. These species are used as curing agents in epoxy resins.[16] Another market for maleic anhydride is lubricating oil additives, which are used in gasoline and diesel engine crankcase oils as dispersants and corrosion inhibitors. Changes in lubricant specifications and more efficient engines have had a negative effect on the demand for lubricating oil additives, giving flat growth prospects for maleic anhydride in this application.
Others
A number of smaller applications exist for maleic anhydride. Personal care products consuming maleic anhydride include hair sprays, adhesives and floor polishes. Maleic anhydride is also a precursor to compounds used for water treatment detergents, insecticides and fungicides, pharmaceuticals, and other copolymers.
The maleic anhydride group occurs in several natural products, some of which show promising therapeutic or pesticidal activity.[17] In the wood science, maleic anhydride in combination with other agents, has been used for protection and modification of wood in order to improve its material properties.[18][19]
Production By Region
| Region | 2015 |
|---|---|
| North America | 370 kt (410,000 short tons) |
| South & Central America | 46 kt (51,000 short tons) |
| Western Europe | 307 kt (338,000 short tons) |
| Central & Eastern Europe | 60 kt (66,000 short tons) |
| Asia | 1,864 kt (2,055,000 short tons) |
| Africa | 14 kt (15,000 short tons) |
| Total | 2,771 kt (3,055,000 short tons) |
Packing and transport
Template:Unref section Liquid maleic anhydride is available in road tankers and/or tank-containers which are made of stainless steel, which are insulated and provided with heating systems to maintain the temperature of 65–75 °C (149–167 °F). Tank cars must be approved for the transport of molten maleic anhydride.
Liquid/molten maleic anhydride is a dangerous material in accordance with RID/ADR.
Solid maleic anhydride pellets are transported by trucks. Packaging is generally in 25 kg (55 lb) polyethylene bags.
Effects on human health and the environment
This compound poses relatively low-risk environmental hazards, an important feature for some applications. In humans, exposure to maleic anhydride may cause irritation to the respiratory tract, eyes, exposed mucosa, and skin. Maleic anhydride is also a skin and respiratory sensitizer.[20]
Maleic anhydride is a low hazard profile chemical. Maleic anhydride rapidly hydrolyzes to form maleic acid in the presence of water and hence environmental exposures to maleic anhydride itself are unlikely. Maleic acid is biodegradable under aerobic conditions in sewage sludge as well as in soil and water.
Food starch for use in night markets sold from a supplier in Tainan city, Taiwan, were found to contain maleic anhydride in December 2013. The supplier was investigated regarding the 300 t (330 short tons) of tainted starch; an earlier inspection in November had found 32 t (35 short tons).[21]
References
- ↑ Merck Index, 11th Edition, 5586.
- ↑ 2.0 2.1 "Front Matter". Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 835. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 NIOSH Pocket Guide to Chemical Hazards. "#0376". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0376.html.
- ↑ "Maleic anhydride". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/108316.html.
- ↑ 5.0 5.1 Kurt Lohbeck; Herbert Haferkorn; Werner Fuhrmann; Norbert Fedtke (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a16_053.
- ↑ Roux, María Victoria; Jiménez, Pilar; Martín-Luengo, Maria Ángeles; Dávalos, Juan Z.; Sun, Zhiyuan; Hosmane, Ramachandra S.; Liebman, Joel F. (May 1997). "The Elusive Antiaromaticity of Maleimides and Maleic Anhydride: Enthalpies of Formation of N-Methylmaleimide, N-Methylsuccinimide, N-Methylphthalimide, and N-Benzoyl-N-methylbenzamide" (in en). The Journal of Organic Chemistry 62 (9): 2732–2737. doi:10.1021/jo9621985. ISSN 0022-3263. PMID 11671632.
- ↑ Philipp, Bertram; Stevens, Peter (1987). Grundzüge der industriellen Chemie: ein einführendes Lehr- u. Lernbuch. Weinheim: VCH-Verl.-Ges. p. S. 179. ISBN 3-527-25991-0.
- ↑ 8.0 8.1 Hood, David K. (2024). "Maleic Anhydride, Maleic Acid, and Fumaric Acid". Kirk-Othmer Encyclopedia of Chemical Technology. pp. 1–51. doi:10.1002/0471238961.1301120506051220.a01.pub3. ISBN 978-0-471-48494-3.
- ↑ Samuel Danishefsky; Takeshi Kitahara; Paul F. Schuda (1983). "Preparation and Diels-Alder Reaction of a Highly Nucleophilic Diene: trans-1-Methoxyl-3-Trimethylsiloxy-1,3-Butadiene and 5β-Methoxycyclohexan-1-one-3β,4β-Dicarboxylic acid Andhydride". Org. Synth. 61: 147. doi:10.1002/0471264180.os061.30. ISBN 978-0-471-26422-4.
- ↑ & Kurt Alder"Organic compound having hydrogenated ring systems and process of preparing it" StatesUS1944731A United States patent Expired US1944731A, published January 23, 1934, issued 01-23-1934
- ↑ "Reaction of maleic anhydride with active methylene or methine containing compounds" StatesUS4146543A United States patent Expired US4146543A, published 03-27-1979, issued 03-27-1979, assigned to Lever Brothers Company, New York, N.Y.
- ↑ & V.Lamberti"Preparation of cis and trans aconitic acids and their salts" StatesUS4123458A United States patent Expired US4123458A, published 10-31-1978, issued 10-31-1978, assigned to Lever Brothers Company, New York, N.Y.
- ↑ Horie, T.; Sumino, M.; Tanaka, T.; Matsushita, Y.; Ichimura, T.; Yoshida, J.I. (2010). "Photodimerization of Maleic Anhydride in a Microreactor Without Clogging". Organic Process Research & Development 14 (2): 405–410. doi:10.1021/op900306z.
- ↑ Weiss, E.; Stark, K.; Lancaster, J. E.; Murdoch, H. D. (1963). "π-Olefin-eisentetracarbonyl-Komplexe mit Liganden der Malein-, Fumar-, Acryl-, Methacryl- und Zimtsäure-Reihe". Helvetica Chimica Acta 46: 288–297. doi:10.1002/hlca.19630460128.
- ↑ AP-42: Compilation of Air Emissions Factors from Stationary Sources (Report). U.S. Environmental Protection Agency. 1983.
- ↑ Hara, Osamu (December 20, 1990). "Technical News #32: Curing Agents for Epoxy Resin". https://www.threebond.co.jp/en/technical/technicalnews/pdf/tech32.pdf.
- ↑ Chen, Xiaolong; Zheng, Yuguo; Shen, Yinchu (2007). "Natural Products with Maleic Anhydride Structure: Nonadrides, Tautomycin, Chaetomellic Anhydride, and Other Compounds". Chemical Reviews 107 (5): 1777–1830. doi:10.1021/cr050029r. PMID 17439289.
- ↑ Zheng, Dingyuan; Li, Zehuai; Yao, Wenrui; Wang, Yuning; Sun, Ce; Tan, Haiyan; Zhang, Yanhua (2024). "A maleic anhydride-mediated green and sustainable route for versatile wood platform". Chemical Engineering Journal (Elsevier BV) 479. doi:10.1016/j.cej.2023.147907. ISSN 1385-8947.
- ↑ Kim, Injeong; Ross, Lone; Alfredsen, Gry; Karlsson, Olov; Kaynak, Elif; Das, Oisik; Jones, Dennis; Mantanis, George I. et al. (2025-03-16). "Enhancement of Biological Durability and Fire Safety in Wood Modified with Maleic Anhydride and Sodium Hypophosphite". Forests (MDPI AG) 16 (3): 526. doi:10.3390/f16030526. ISSN 1999-4907. http://urn.kb.se/resolve?urn=urn:nbn:se:ltu:diva-112064.
- ↑ "Substance Evaluation Report: Maleic anhydride". Environment Agency Austria. http://echa.europa.eu/documents/10162/9801478/sev1_203_571_6_report_en.pdf.
- ↑ "Tainted starch found in Tainan yet again". Want China Times. 2013-12-19. http://www.wantchinatimes.com/news-subclass-cnt.aspx?id=20131219000005&cid=1103.
External links
- International Chemical Safety Card 0799
- NIOSH Pocket Guide to Chemical Hazards. "#0376". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0376.html.
- Chronic toxicity summary
- Maleic anhydride at Occupational Safety & Health Administration
 |
 KSF
KSF