Malvin
Topic: Chemistry
 From HandWiki - Reading time: 6 min
From HandWiki - Reading time: 6 min
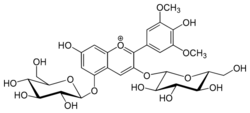
| |
| Names | |
|---|---|
| IUPAC name
3,5-Bis(β-D-glucopyranosyloxy)-4′,7-dihydroxy-3′,5′-dimethoxyflavylium
| |
| Systematic IUPAC name
7-Hydroxy-2-(4-hydroxy-3,5-dimethoxyphenyl)-3,5-bis{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-1λ4-benzopyran-1-ylium | |
| Other names
Malvidin 3,5-diglucoside
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| |
| Molar mass |
|
| Appearance | Reddish blue, odorless powder[1] |
| Nearly insoluble[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Malvin is a naturally occurring chemical of the anthocyanin family.
Malvin reacts in the presence of H2O2 to form malvone.[2] The ortho-benzoyloxyphenylacetic acid esters reaction product is dependant of the pH: it is obtained under acidic conditions whereas under neutral conditions, the reaction product is the 3-O-acyl-glucosyl-5-O-glucosyl-7-hydroxy coumarin.[3]
Natural occurrences
It is a diglucoside of malvidin mainly found as a pigment in herbs like Malva (Malva sylvestris), Primula and Rhododendron.[4] M. sylvestris also contains malonylmalvin (malvidin 3-(6″-malonylglucoside)-5-glucoside).[5]
The characteristic floral jade coloration of Strongylodon macrobotrys has been shown to be an example of copigmentation, a result of the presence of malvin and saponarin (a flavone glucoside) in the ratio 1:9.
Presence in food
Malvin can be found in a variety of common foods, including peaches (Clingstone variety[6]).
References
- ↑ 1.0 1.1 MSDS from CarlRoth (German)
- ↑ Oxidation of the anthocyanidin-3,5-diglucosides with H2O2: The structure of malvone. G. Hrazdina, Phytochemistry, July 1970, Volume 9, Issue 7, Pages 1647–1652, doi:10.1016/S0031-9422(00)85290-5
- ↑ Oxidation products of acylated anthocyanins under acidic and neutral conditions. Géza Hrazdina and Angeline J. Franzese, Phytochemistry, January 1974, Volume 13, Issue 1, Pages 231–234, doi:10.1016/S0031-9422(00)91300-1
- ↑ J. A. Joule, K. Mills: Heterocyclic Chemistry., S. 173, Blackwell Publishing, 2000, ISBN 978-0-632-05453-4
- ↑ Malonated anthocyanins in malvaceae: Malonylmalvin from Malva sylvestris. Kosaku Takeda, Shigeki Enoki, Jeffrey B. Harborne and John Eagles, Phytochemistry, 1989, Volume 28, Issue 2, Pages 499–500, doi:10.1016/0031-9422(89)80040-8
- ↑ Chang, S; Tan, C; Frankel, EN; Barrett, DM (2000). "Low-density lipoprotein antioxidant activity of phenolic compounds and polyphenol oxidase activity in selected clingstone peach cultivars". Journal of Agricultural and Food Chemistry 48 (2): 147–51. doi:10.1021/jf9904564. PMID 10691607.
 |
 KSF
KSF