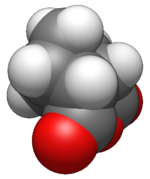
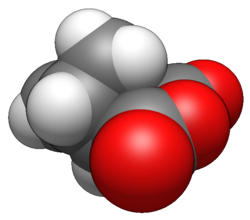
Nadic anhydride
Topic: Chemistry
 From HandWiki - Reading time: 2 min
From HandWiki - Reading time: 2 min

| |||
| |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name
24-oxatricyclo[5.2.1.02,6]dec-8-ene-3,5-dione | |||
| Other names
5-Norbornene-2,3-dicarboxylic anhydride
Nadic acid anhydride Himic anhydride (exo) Carbic anhydride (endo) | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| C9H8O3 | |||
| Molar mass | 164.160 g·mol−1 | ||
| Appearance | white solid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Nadic anhydride, also known as 5-norbornene-2,3-dicarboxylic anhydride, is an organic acid anhydride derivative of norbornene.
Stereochemistry
Nadic anhydride exhibits endo-exo isomerism. In the exo isomer, the acid anhydride group points in the same direction towards the bridging carbon of the norbornene, while in the endo isomer the acid anhydride group points in the opposite direction. These isomers are respectively named cis-5-norbornene-exo-2,3-dicarboxylic anhydride (also known as himic anhydride) and cis-5-norbornene-endo-2,3-dicarboxylic anhydride (also known as carbic anhydride).[1][2] Commercially available nadic anhydride is mainly the endo isomer, as this is the isomer predominantly made in the Diels-Alder reaction in its synthesis.[3]
 |

|
 |

|
| endo | exo |
Preparation
In the patent for the Diels-Alder reaction, nadic anhydride was given as an example of the reaction, made by the condensation of maleic anhydride and cyclopentadiene, which gives mostly the endo isomer.[3] The endo isomer can be converted into the exo isomer by irradiation with UV light.[4]
Uses
Due to the reactivity of the norbornene moiety in the thiol-ene reaction, nadic anhydride is used in the synthesis of monomers for cross-linked polymer networks based on thiol-ene linkages.[5]
References
- ↑ Template:Cite PubChem
- ↑ Template:Cite PubChem
- ↑ 3.0 3.1 & Kurt Alder"Organic compound having hydrogenated ring systems and process of preparing it" StatesUS1944731A United States patent US1944731A, published January 23, 1934
- ↑ Pandey, Bipin; Athawale, Asawar A.; Reddy, Ravinder S.; Dalvi, Pravinder S.; Kumar, Pradeep (1991). "A Remarkably Efficient Photochemical Methodology for Endo to Exo IsomerizatLon of Dials–Alder Cycloadducts". Chemistry Letters 20 (7): 1173–1176. doi:10.1246/cl.1991.1173.
- ↑ Hoyle, Charles E.; Bowman, Christopher N. (2010). "Thiol–Ene Click Chemistry". Angewandte Chemie International Edition 49 (9): 1540–1573. doi:10.1002/anie.200903924.
 |
 KSF
KSF