Niobium monoxide
Topic: Chemistry
 From HandWiki - Reading time: 5 min
From HandWiki - Reading time: 5 min
| |
| Names | |
|---|---|
| Other names
niobium(II) oxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| NbO | |
| Molar mass | 108.905 g/mol[1] |
| Appearance | grey solid[1] |
| Odor | odorless |
| Density | 7.30 g/cm3[1] |
| Melting point | 1,937 °C (3,519 °F; 2,210 K)[1] |
| Solubility | slightly soluble in HCl insoluble in nitric acid |
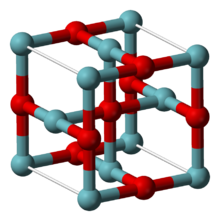
| Structure[2] | |
| Cubic, cP6 | |
| Pm3m, No. 221 | |
a = 0.4211 nm
| |
Formula units (Z)
|
3 |
| Thermochemistry[3] | |
Heat capacity (C)
|
41.3 J/(mol·K) |
Std molar
entropy (S |
48.1 J/(mol·K) |
Std enthalpy of
formation (ΔfH⦵298) |
= -405.85 kJ/mol |
Gibbs free energy (ΔfG˚)
|
-378.6 kJ/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Niobium monoxide is the inorganic compound with the formula NbO. It is a grey solid with metallic conductivity.[1]
Structure and electronic properties
NbO adopts an unusual cubic structure, similar to the rock salt structure but with some missing atoms compared to it, so that both niobium and oxygen atoms have square planar coordination geometries. The niobium centers are arranged in octahedra, and there is a structural similarity to the octahedral niobium clusters in lower halides of niobium. In NbO the Nb-Nb bond length is 298 pm which compares to 285 pm in the metal.[2] One study of the bonding concludes that strong and nearly covalent bonds exist between the metal centers.[4]
It is a superconductor with a transition temperature of 1.38 K.[5] It is used in capacitors where a layer of Nb2O5 is formed around NbO grains as the dielectric.[6][7][8]
Preparation
NbO can be prepared by reduction of Nb2O5 by H2. More typically, it is prepared by comproportionation:[9]
- Nb2O5 + 3 Nb → 5 NbO
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Haynes, p. 4.76
- ↑ 2.0 2.1 Pialoux, A.; Joyeux, M.L; Cizeron, G. (1982). "Étude du comportement du niobium sous vide par diffraction des rayons X à haute température". Journal of the Less Common Metals 87: 1–19. doi:10.1016/0022-5088(82)90036-4.
- ↑ Haynes, p. 5.29
- ↑ Schulz, Werner W.; Wentzcovitch, Renata M. (1993). "Electronic band structure and bonding in Nb3O3". Physical Review B 48 (23): 16986–16991. doi:10.1103/PhysRevB.48.16986. PMID 10008298. Bibcode: 1993PhRvB..4816986S.
- ↑ Hulm, J. K.; Jones, C. K.; Hein, R. A.; Gibson, J. W. (1972). "Superconductivity in the TiO and NbO systems". Journal of Low Temperature Physics 7 (3–4): 291–307. doi:10.1007/BF00660068. Bibcode: 1972JLTP....7..291H.
- ↑ Nico, C.; Soares, M. R. N.; Rodrigues, J.; Matos, M.; Monteiro, R.; Graça, M. P. F.; Valente, M. A.; Costa, F. M. et al. (2011). "Sintered NbO Powders for Electronic Device Applications". The Journal of Physical Chemistry C 115 (11): 4879–4886. doi:10.1021/jp110672u.
- ↑ Nico, Cláudio; Rino, Luís; Matos, Mariana; Monteiro, Rui; Costa, Florinda M.; Monteiro, Teresa; Graça, Manuel P.F. (2013). "NbO/Nb2O5 core–shells by thermal oxidation". Journal of the European Ceramic Society 33 (15–16): 3077–3083. doi:10.1016/j.jeurceramsoc.2013.06.020.
- ↑ Naito, Kazumi and Kabe, Isao (2005) "Production method of solid electrolytic capacitor" U.S. Patent 6,882,522
- ↑ Reed, T. B.; Pollard, E. R.; Lonney, L. E.; Loehman, R. E.; Honig, J. M. (2007). "Niobium Monoxide". Inorganic Syntheses. pp. 108–110. doi:10.1002/9780470132616.ch22. ISBN 9780470132616.
Cited sources
 |
 KSF
KSF