Nitrone
Topic: Chemistry
 From HandWiki - Reading time: 2 min
From HandWiki - Reading time: 2 min
In organic chemistry, a nitrone is a functional group consisting of an N-oxide of an imine. The general structure is R1
R2
C=N+
(–O−
)(–R3
), where R3 is not a hydrogen. Their primary application is intermediates in chemical synthesis. A nitrone is a 1,3-dipole used in cycloadditions, and a carbonyl mimic.
Structure
Nitrones, as a tetrasubstituted double bond, admit cis–trans isomerism.[1]: 474
Generation of nitrones
Typical nitrone sources are hydroxylamine oxidation or condensation with carbonyl compounds. Secondary hydroxylamines oxidize to nitrones in air over a timescale of several weeks, a process cupric salts accelerate.[1]: 476 [2]: 332–333 The most general reagent used for the oxidation of hydroxylamines is aqueous mercuric oxide:[1]: 476 [3]
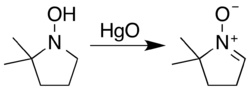
However, a hydroxylamine with two α hydrogens may unsaturate on either side. Carbonyl condensation avoids this ambiguity...[4]center ...but is inhibited if both ketone substituents are bulky.[1]: 477
In principle, N-alkylation could produce nitrones from oximes, but in practice electrophiles typically perform a mixture of N- and O-attack.[1]: 479 [2]: 334
Reactions
Some nitrones oligomerize:[1]: 483 [2]: 334,337-338 [5] centerSyntheses with nitrone precursors obviate the issue with increased temperature, to exaggerate entropic factors; or with a nitrone excess.
Carbonyl mimic
Like many other unsaturated functional groups, nitrones activate the α and β carbons towards reaction. The α carbon is an electrophile and the β carbon a nucleophile; that is, nitrones polarize like carbonyls and nitriles but unlike nitro compounds and vinyl sulfur derivatives.[1]: 483 [2]: 338–340
Nitrones hydrolyze extremely easily to the corresponding carbonyl and N-hydroxylamine.[1]: 491 [2]: 344
1,3-dipolar cycloadditions
As 1,3‑dipoles, nitrones perform [3+2] cycloadditions.[6] For example, a dipolarophilic alkene combines to form isoxazolidine:
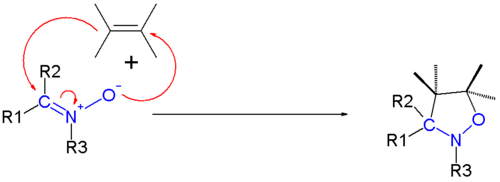
Other ring-closing reactions are known,[7] including formal [3+3] and [5+2] cycloadditions.[6]
Isomerization
Deoxygenating reagents, light, or heat all catalyze rearrangement to the amide. Acids catalyze rearrangement to the oxime ether.[1]: 489–490 [2]: 345–347
Reduction
Hydrides add to give hydroxylamines. Reducing Lewis acids (e.g. metals, SO
2) deoxygenate to the imine instead.[1]: 490 [2]: 343
See also
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 Hamer, Jan; Macaluso, Anthony (1964-08-01). "Nitrones" (in en). Chemical Reviews 64 (4): 473–495. doi:10.1021/cr60230a006. ISSN 0009-2665. https://pubs.acs.org/doi/abs/10.1021/cr60230a006.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Delpierre, G. R.; Lamchen, M. (1965). "Nitrones" (in en). Quarterly Reviews, Chemical Society 19 (4): 329. doi:10.1039/qr9651900329. ISSN 0009-2681. http://xlink.rsc.org/?DOI=qr9651900329.
- ↑ Thiesing, Jan; Mayer, Hans (1957). "Cyclische Nitrone, II. Über die Polymeren des 2.3.4.5-Tetrahydro-pyridin-N-oxyds und verwandte Verbindungen". Justus Liebigs Ann. Chem. 609: 46-57. doi:10.1002/jlac.19576090105.
- ↑ Exner, O. (1951). "A New Synthesis of N-methylketoximes". ChemPlusChem 16: 258-267. doi:10.1135/cccc19510258.
- ↑ Thiesing, Jan; Mayer, Hans (1956). "Cyclische Nitrone I: Dimeres 2.3.4.5-Tetrahydro-pyridin-N-oxyd". Chem. Ber. 89 (9): 2159-2167. doi:10.1002/cber.19560890919.
- ↑ 6.0 6.1 Yang, Jiong (2012). "Recent Developments in Nitrone Chemistry". Synlett 23: 2293-97. doi:10.1055/s-0032-1317096.
- ↑ Murahashi, Shun-Ichi; Imada, Yasushi (15 March 2019). "Synthesis and Transformations of Nitrones for Organic Synthesis". Chemical Reviews 119 (7): 4684–4716. doi:10.1021/acs.chemrev.8b00476. PMID 30875202.
 |
 KSF
KSF