Nutlin
Topic: Chemistry
 From HandWiki - Reading time: 3 min
From HandWiki - Reading time: 3 min
| |
| Names | |
|---|---|
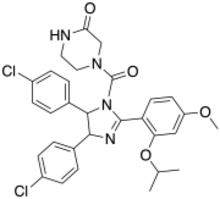
| IUPAC name
(±)-4-[4,5-Bis(4-chlorophenyl)-2-(2-isopropoxy-4-methoxy-phenyl)-4,5-dihydro-imidazole-1-carbonyl]-piperazin-2-one
| |
| Other names
Nutlin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
PubChem CID
|
|
| UNII | |
| |
| Properties | |
| C30H30Cl2N4O4 | |
| Molar mass | 581.49 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nutlins are cis-imidazoline analogs which inhibit the interaction between mdm2 and tumor suppressor p53, and which were discovered by screening a chemical library by Vassilev et al. Nutlin-1, nutlin-2, and nutlin-3 were all identified in the same screen;[1] however, Nutlin-3 is the compound most commonly used in anti-cancer studies.[2] Nutlin small molecules occupy p53 binding pocket of MDM2 and effectively disrupt the p53–MDM2 interaction that leads to activation of the p53 pathway in p53 wild-type cells.[3] Inhibiting the interaction between mdm2 and p53 stabilizes p53, and is thought to selectively induce a growth-inhibiting state called senescence in cancer cells. These compounds are therefore thought to work best on tumors that contain normal or "wild-type" p53.[citation needed] Nutlin-3 has been shown to affect the production of p53 within minutes.[4]
The more potent of the two enantiomers, nutlin-3a ((–)-nutlin-3), can be synthesized in a highly enantioselective fashion.[5] Several derivatives of nutlin, such as RG7112 and RG7388 (Idasanutlin) have been developed and progressed into human studies.[6] Imidazoline core based on the methoxyphenyl substituents also stabilizes p53.[7][8][9]
References
- ↑ "In vivo activation of the p53 pathway by small-molecule antagonists of MDM2". Science 303 (5659): 844–8. February 2004. doi:10.1126/science.1092472. PMID 14704432. Bibcode: 2004Sci...303..844V.
- ↑ "Small-molecule inhibitors of the MDM2-p53 protein-protein interaction to reactivate p53 function: a novel approach for cancer therapy". Annual Review of Pharmacology and Toxicology 49: 223–41. 2008. doi:10.1146/annurev.pharmtox.48.113006.094723. PMID 18834305.
- ↑ "Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy". Proceedings of the National Academy of Sciences of the United States of America 103 (6): 1888–93. February 2006. doi:10.1073/pnas.0507493103. PMID 16443686.
- ↑ "Mechanism-specific signatures for small-molecule p53 activators". Cell Cycle (Landes Bioscience) 10 (10): 1590–8. May 2011. doi:10.4161/cc.10.10.15519. PMID 21490429.
- ↑ "Catalytic, Enantioselective Synthesis of Stilbene cis-Diamines: A Concise Preparation of (-)-Nutlin-3, a Potent p53/MDM2 Inhibitor". Chemical Science 2 (6): 1076–1079. January 2011. doi:10.1039/C1SC00061F. PMID 22708054.
- ↑ "Prolonged Idasanutlin (RG7388) Treatment Leads to the Generation of p53-Mutated Cells". Cancers (Basel) 10 (11): 396. Nov 2018. doi:10.3390/cancers10110396. PMID 30352966.
- ↑ Bazanov, Daniil R.; Pervushin, Nikolay V.; Savin, Egor V.; Tsymliakov, Michael D.; Maksutova, Anita I.; Sosonyuk, Sergey E.; Kopeina, Gelina S.; Lozinskaya, Natalia A. (December 2021). "Sulfonamide derivatives of cis-imidazolines as potent p53-MDM2/MDMX protein-protein interaction inhibitors" (in en). Medicinal Chemistry Research 30 (12): 2216–2227. doi:10.1007/s00044-021-02802-w. ISSN 1054-2523. https://link.springer.com/10.1007/s00044-021-02802-w.
- ↑ Bazanov, Daniil R.; Pervushin, Nikolay V.; Savitskaya, Victoria Yu.; Anikina, Lada V.; Proskurnina, Marina V.; Lozinskaya, Natalia A.; Kopeina, Gelina S. (August 2019). "2,4,5-Tris(alkoxyaryl)imidazoline derivatives as potent scaffold for novel p53-MDM2 interaction inhibitors: Design, synthesis, and biological evaluation" (in en). Bioorganic & Medicinal Chemistry Letters 29 (16): 2364–2368. doi:10.1016/j.bmcl.2019.06.007. PMID 31196710. https://linkinghub.elsevier.com/retrieve/pii/S0960894X19303749.
- ↑ Bazanov, Daniil R.; Pervushin, Nikolay V.; Savin, Egor V.; Tsymliakov, Michael D.; Maksutova, Anita I.; Savitskaya, Victoria Yu.; Sosonyuk, Sergey E.; Gracheva, Yulia A. et al. (2022-04-02). "Synthetic Design and Biological Evaluation of New p53-MDM2 Interaction Inhibitors Based on Imidazoline Core" (in en). Pharmaceuticals 15 (4): 444. doi:10.3390/ph15040444. ISSN 1424-8247. PMID 35455441.
 |
 KSF
KSF