Ononin
Topic: Chemistry
 From HandWiki - Reading time: 4 min
From HandWiki - Reading time: 4 min
| |
| Names | |
|---|---|
| IUPAC name
7-(β-D-Glucopyranosyloxy)-4′-methoxyisoflavone
| |
| Systematic IUPAC name
3-(4-Methoxyphenyl)-7-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-4H-1-benzopyran-4-one | |
| Other names
Formononetin glucoside
Formononetin-7-glucoside Formononetin 7-O-glucoside | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C22H22O9 | |
| Molar mass | 430.409 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
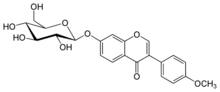
Ononin is an isoflavone glycoside, the 7-O-β-D-glucopyranoside of formononetin,[1] which in turn is the 4'-O-methoxy derivative of the parent isoflavone daidzein.
Natural sources
Ononin is a major isoflavone [2] found in a number of plants and herbs like soybean[3] and Glycyrrhiza uralensis.[4]
Pharmacokinetics
Intestinal bacterial metabolic pathways may include demethylation and deglycosylation.[5] It follows that formation of formononetin and/or daidzein is possible.
Pharmacodynamics
An in vitro anti-inflammatory effect on lipopolysaccharide (LPS)-induced inflammation has been demonstrated in one study.[6]
References
- ↑ You-Ping Zhu (28 May 1998). Chinese Materia Medica: Chemistry, Pharmacology and Applications. p. 622. ISBN 9057022850.
- ↑ Dong, Lin; Yin, Lei; Zhang, Yuanbin; Fu, Xueyan; Lu, Jincai (2017). "Anti-inflammatory effects of ononin on lipopolysaccharide-stimulated RAW 264.7 cells". Molecular Immunology 83: 46–51. doi:10.1016/j.molimm.2017.01.007. PMID 28095349.
- ↑ Stanley F. Osman; William F. Fett (1983). "Isoflavone glucoside stress metabolites of soybean leaves". Phytochemistry 2 (9): 1921–1923. doi:10.1016/0031-9422(83)80013-2.
- ↑ Tsutomu Nakanishi; Akira Inada; Kazuko Kambayashia; Kaisuke Yonedaa (1985). "Flavonoid glycosides of the roots of Glycyrrhiza uralensis". Phytochemistry 24 (2): 339–341. doi:10.1016/S0031-9422(00)83548-7.
- ↑ Zhang W, Jiang S, Qian DW, Shang EX, Guan HL, Ren H, Zhu ZH, Duan JA (2014). "The interaction between ononin and human intestinal bacteria" (in Chinese). Yao Xue Xue Bao 49 (8): 1162–1168. PMID 25322559.
- ↑ Dong, Lin; Yin, Lei; Zhang, Yuanbin; Fu, Xueyan; Lu, Jincai (2017). "Anti-inflammatory effects of ononin on lipopolysaccharide-stimulated RAW 264.7 cells". Molecular Immunology 83: 46–51. doi:10.1016/j.molimm.2017.01.007. PMID 28095349.
 |
Licensed under CC BY-SA 3.0 | Source: https://handwiki.org/wiki/Chemistry:Ononin10 views | ↧ Download this article as ZWI file
 KSF
KSF