PIPES
Topic: Chemistry
 From HandWiki - Reading time: 4 min
From HandWiki - Reading time: 4 min
| |
| Names | |
|---|---|
| Preferred IUPAC name
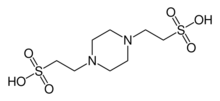
2,2′-(Piperazine-1,4-diyl)di(ethane-1-sulfonic acid) | |
| Other names
PIPES
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H18N2O6S2 | |
| Molar mass | 302.37 |
| Appearance | White powder |
| Melting point | Decomposes above 300 °C |
| Boiling point | Decomposes |
| 1 g/L (100 °C) | |
| Hazards | |
| Main hazards | Irritant |
| Safety data sheet | External MSDS |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
PIPES (piperazine-N,N′-bis(2-ethanesulfonic acid)) is a frequently used buffering agent in biochemistry. It is an ethanesulfonic acid buffer developed by Good et al. in the 1960s.[1]
Applications
PIPES has two pKa values. One pKa (6.76 at 25 °C) is near the physiological pH which makes it useful in cell culture work. Its effective buffering range is 6.1-7.5 at 25 °C. The second pKa value is at 2.67 with a buffer range of from 1.5-3.5. PIPES has been documented minimizing lipid loss when buffering glutaraldehyde histology in plant and animal tissues.[2][3] Fungal zoospore fixation for fluorescence microscopy and electron microscopy were optimized with a combination of glutaraldehyde and formaldehyde in PIPES buffer.[4] It has a negligible capacity to bind divalent ions.[5]
See also
References
- ↑ Good, Norman E.; Winget, G. Douglas; Winter, Wilhelmina; Connolly, Thomas N.; Izawa, Seikichi; Singh, Raizada M. M. (1966). "Hydrogen Ion Buffers for Biological Research". Biochemistry 5 (2): 467–77. doi:10.1021/bi00866a011. PMID 5942950.
- ↑ Salema, R. and Brando, I., J. Submicr. Cytol., 9, 79 (1973).
- ↑ Schiff, R.I. and Gennaro, J.F., Scaning Electron Microsc., 3, 449 (1979).
- ↑ Hardham, A.R. (1985). "Studies on the cell surface of zoospores and cysts of the fungus Phytophthora cinnamomi: The influence of fixation on patterns of lectin binding". Journal of Histochemistry 33 (2): 110–8. doi:10.1177/33.2.3918095. PMID 3918095. http://www.jhc.org/cgi/content/abstract/33/2/110.
- ↑ "Hopax Fine Chemicals - Biological buffers and their interactions with metal ions". https://www.hopaxfc.com/en/blog/biological-buffers-and-their-interactions-with-metal-ions.
 |
 KSF
KSF
