Palladium(II,IV) fluoride
Topic: Chemistry
 From HandWiki - Reading time: 5 min
From HandWiki - Reading time: 5 min
| |
| Names | |
|---|---|
| Other names
palladium(II) hexafluoropalladate(IV)
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| F3Pd | |
| Molar mass | 163.42 g·mol−1 |
| Appearance | black solid |
| +1760.0·10−6 cm3/mol | |
| Structure | |
| rhombohedral | |
| octahedral | |
| Related compounds | |
Other cations
|
Nickel(III) fluoride |
Related compounds
|
Palladium(II) fluoride Palladium(IV) fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Palladium(II,IV) fluoride, also known as palladium trifluoride, is a chemical compound of palladium and fluorine. It has the empirical formula PdF3, but is better described as the mixed-valence compound palladium(II) hexafluoropalladate(IV), PdII[PdIVF6], and is often written as Pd[PdF6] or Pd2F6.[1][2]
Synthesis
Pd[PdF6] is the most stable product of the reaction of fluorine and metallic palladium.[1]
- 2 Pd + 3 F2 → Pd[PdF6]
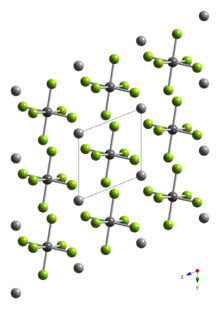
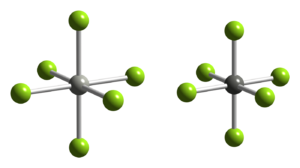
Structure and properties
Pd[PdF6] is paramagnetic, and both Pd(II) and Pd(IV) occupy octahedral sites in the crystal structure.[2][3] The PdII-F distance is 2.17 Å, whereas the PdIV-F distance is 1.90 Å.[4]
See also
References
- ↑ 1.0 1.1 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 1152–1153. ISBN 978-0-08-037941-8.
- ↑ 2.0 2.1 Housecroft, C. E.; Sharpe, A. G. (2008). Inorganic Chemistry (3rd ed.). Prentice Hall. p. 788. ISBN 978-0-13-175553-6.
- ↑ Hepworth, M. A.; Jack, K. H.; Peacock, R. D.; Westland, G. J. (1957). "The crystal structures of the trifluorides of iron, cobalt, ruthenium, rhodium, palladium and iridium". Acta Crystallogr. 10: 63–69. doi:10.1107/S0365110X57000158.
- ↑ Tressaud, A.; Bartlett, N. (2001). "Preparation, Magnetic Properties, and Pressure-Induced Transitions of Some MIIMIVF6 (MII=Ni, Pd, Cu; MIV=Pd, Pt, Sn) Complex Fluorides". J. Solid State Chem. 162 (2): 333–340. doi:10.1006/jssc.2001.9331. Bibcode: 2001JSSCh.162..333T.
 |
Licensed under CC BY-SA 3.0 | Source: https://handwiki.org/wiki/Chemistry:Palladium(II,IV)_fluoride12 views | ↧ Download this article as ZWI file
 KSF
KSF