Penam
Topic: Chemistry
 From HandWiki - Reading time: 5 min
From HandWiki - Reading time: 5 min
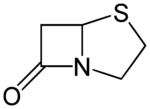
| |
| Names | |
|---|---|
| IUPAC name
(5R)-4-thia-1-azabicyclo[3.2.0]heptan-7-one
| |
| Other names
1-Aza-7-oxo-4-thiabicyclo[3.2.0]heptane
| |
| Identifiers | |
3D model (JSmol)
|
|
| 4374479 | |
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C5H7NOS | |
| Molar mass | 129.18 g·mol−1 |
| Related compounds | |
Related compounds
|
clavam |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
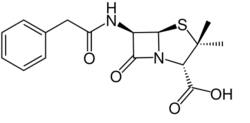
Penams are the primary skeleton structures that define the penicillin subclass of the broader β-lactam family of antibiotics and related compounds. They are bicyclic ring systems containing a β-lactam moiety fused with a five-member thiazolidine ring. [1] Due to ring strain and limitations on amide resonance, the structure is unstable and highly susceptible to catalytic cleavage at the amide bond.[2] Benzylpenicillin (penicillin G) is the natural product parent that contains the penam structure.
Structure
Penams have inflexible structures. The structure is locked in a puckered (i.e. bent) shape due to the pyramidal geometry of the bridgehead nitrogen. The pyramidalization (χ = 54°) and twist of the C-N bond (τ = 18°) is caused by the strain from the lone pair's exclusion from planarity with the cyclic rings and electrostatic repulsion effects. As a result, the distorted C-N bond causes misalignment the orbitals of the carbonyl carbon and the nitrogen lone pair that allow for resonance overlap. The amide C-N bond length is 1.406 Å and displays greater single bond character than in noncyclic tertiary amides. The C-O bond length is 1.205 Å which is shorter than C-O bonds in noncyclic tertiary amides.[3]
Penams are strained associated with the four-member β-lactam ring, whose internal bond angles are 90º.[4][3] Consequently, penams are susceptible to acid- and base-catalyzed hydrolysis.[1][4]
References
- ↑ 1.0 1.1 Novak, Igor; Chua, Pei Juan (2006-09-01). "Computational Study of Pharmacophores: β-Lactams". The Journal of Physical Chemistry A 110 (35): 10521–10524. doi:10.1021/jp063162b. ISSN 1089-5639. PMID 16942059. Bibcode: 2006JPCA..11010521N.
- ↑ Patrick, Graham (2017-03-23), "5. Pharmaceuticals and medicinal chemistry", Organic Chemistry: A Very Short Introduction (Oxford University Press): pp. 71–89, doi:10.1093/actrade/9780198759775.003.0005, ISBN 978-0-19-875977-5
- ↑ 3.0 3.1 Glover, Stephen A.; Rosser, Adam A. (2012-06-14). "Reliable Determination of Amidicity in Acyclic Amides and Lactams". The Journal of Organic Chemistry 77 (13): 5492–5502. doi:10.1021/jo300347k. ISSN 0022-3263. PMID 22646836.
- ↑ 4.0 4.1 Hu, Feng; Lalancette, Roger; Szostak, Michal (2016-03-08). "Structural Characterization of N-Alkylated Twisted Amides: Consequences for Amide Bond Resonance and N−C Cleavage". Angewandte Chemie International Edition 55 (16): 5062–5066. doi:10.1002/anie.201600919. ISSN 1433-7851. PMID 26953809.
 |
 KSF
KSF