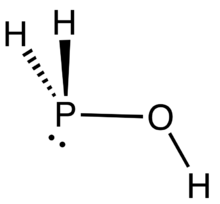
Phosphinous acid
Topic: Chemistry
 From HandWiki - Reading time: 1 min
From HandWiki - Reading time: 1 min
| |
| Names | |
|---|---|
| Other names
hydroxyphosphine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| H3OP | |
| Molar mass | 49.997 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Phosphinous acid (or Phosphinol) is the inorganic compound with the formula H2POH. It exists, fleetingly, as a mixture with its less stable tautomer H3PO (phosphine oxide). This mixture has been generated by low temperature oxidation of phosphine with ozone.[1] H2POH is mainly of pedagogical interest. Organophosphinous acids are more prevalent than the parent H2POH.
Organophosphinous acids
Phosphinous acids exist mainly as minor tautomers of secondary phosphine oxides. For example diphenylphosphinous acid, which is not detectable directly, is invoked as the tautomer of diphenylphosphine oxide.
Highly electron-withdrawing substituents stabilize the phosphinous acid tautomer as illustrated by (CF3)2POH.[2]
References
- ↑ Withnall, Robert; Andrews, Lester (1987). "FTIR spectra of the photolysis products of the phosphine-ozone complex in solid argon". Journal of Physical Chemistry 91 (4): 784–97. doi:10.1021/j100288a008.
- ↑ Bader, Julia; Berger, Raphael J. F.; Stammler, Hans-Georg; Mitzel, Norbert W.; Hoge, Berthold (2011). "First Solid-State Structures of Real Diorganyl Phosphinous Acids R2POH (R=CF3, C2F5)". Chemistry - A European Journal 17 (48): 13420–13423. doi:10.1002/chem.201102370. PMID 22052837.
 |
Licensed under CC BY-SA 3.0 | Source: https://handwiki.org/wiki/Chemistry:Phosphinous_acid8 views | ↧ Download this article as ZWI file
 KSF
KSF