Pisatin
Topic: Chemistry
 From HandWiki - Reading time: 5 min
From HandWiki - Reading time: 5 min
| |
| Names | |
|---|---|
| Preferred IUPAC name
(6aR,12aR)-3-Methoxy-6H,9H-[1,3]dioxolo[4′,5′:5,6][1]benzofuro[3,2-c][1]benzopyran-6a(12aH)-ol | |
| Other names
(+)-Pisatin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C17H14O6 | |
| Molar mass | 314.293 g·mol−1 |
| Related compounds | |
Related compounds
|
anhydropisatin, (−)-maackiain, calycosin |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
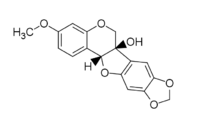
Pisatin (3-hydroxy-7-methoxy-4′,5′-methylenedioxy-chromanocoumarane) is the major phytoalexin made by the pea plant Pisum sativum.[1] It was the first phytoalexin to be purified[2] and chemically identified.[3] The molecular formula is C17H14O6.
Structure and properties
The structure of pisatin consists of a pterocarpan backbone and is distinguishable by the hydroxyl group on the nonaromatic portion of the molecule.[1] This molecule is slightly soluble in water and has high solubility in organic solvents. Pisatin is stable in neutral or slightly basic solutions and loses water in the presence of acid to form anhydropisatin.[4]
Resistance to Pisatin
Resistance to pisatin appears to be an important trait for pathogens of Pisum sativum. Detoxification involves the removal of the 3-O-methyl group, which has been shown to reduce the toxicity of the molecule. An enzyme known as pisatin demethylase is responsible for this catalysis and has been identified in N. haematococca as a cytochrome P450 enzyme. Most fungi capable of this metabolism are resistant to pisatin, however, there are some pathogens that do not contain the gene for pisatin demethylase. Such pathogens may have alternative methods for metabolizing phytoalexins. In addition, many microbial species have been found to have the ability to detoxify pisatin, but the most virulent strains have the highest rate of demethylation.[5]
Known resistant fungi
- N. haematococca[6][7]
- Ascochyta pisi[8]
- Fusarium oxysporum[9]
- Phoma pinodella[10]
- Mycosphaerella pinodes [10]
- Rhizoctonia solani [10]
Biosynthesis
The biosynthesis of pisatin begins with the amino acid L-phenylalanine. A deamination reaction then produces trans-cinnamate,[11] which undergoes hydroxylation to form 4-coumarate.[12] Acetyl-CoA is then added to form 4-coumaryl-CoA.[13] Three malonyl-CoA moities are then added and cyclized to introduce a phenol ring.[14] An isomerization reaction then occurs,[15] followed by a hydroxylation and rearrangement[16] of the phenol group to form 2,4′,7-trihydroxyisoflavonone. This molecule can then follow one of two paths, both of which include the loss of water[17] and a methylation[18][19] to produce formononetin. This product then undergoes hydroxylation to form calycosin,[20] followed by the formation of a dioxolane ring.[21] Another hydroxylation then occurs, followed by an isomerization to form (−)-sopherol.[22] The reduction of a carbonyl to a hydroxyl group [23] and the loss of water [24] then forms (+)-maackiain, which undergoes stereochemical rearrangement and hydroxylation to form (+)-6a-hydroxymaackiain.[25] This molecule is then methylated to yield pisatin.[26][27]
References
- ↑ 1.0 1.1 Cruickshank, Iam (1962). Studies on phytoalexins IV: The antimicrobial spectrum of pisatin.
- ↑ Cruickshank, Iam; Perrin, D.R. (1960). "Isolation of a phytoalexin from Pisum sativum L.". Nature 187 (4739): 799–800. doi:10.1038/187799b0. PMID 13813085. Bibcode: 1960Natur.187..799C.
- ↑ Perrin, D.R.; Bottomley, W. (1962). "Studies on phytoalexins. V. The structure of pisatin from Pisum sativum L.". J. Am. Chem. Soc. 84 (10): 1919–22. doi:10.1021/ja00869a030.
- ↑ Perrin, Dawn R.; Bottomley, W. (1962). "Studies on Phytoalexins. V. The Structure of Pisatin from Pisum sativum L.". Journal of the American Chemical Society 84 (10): 1919–1922. doi:10.1021/ja00869a030.
- ↑ VanEtten, H.D.; Matthews, D.E.; Matthews, P.S. (1989). "Phytoalexin detoxification: Importance for pathogenicity and practical implications". Annual Review of Phytopathology 27: 143–164. doi:10.1146/annurev.phyto.27.1.143. PMID 20214490.
- ↑ VanEtten, H.D.; Matthews, D.E.; Smith, D.A. (1982). "Metabolism of phytoalexins". Phytochemistry 21: 1023–1028. doi:10.1016/s0031-9422(00)82409-7.
- ↑ VanEtten, H.D.; Pueppke, S.G. (1976). "Isoflavonoid phytoalexins, In Biochemcial Aspects of Plant-Parasitic Relationships". Annu. Proc. Phytochem. Soc 13: 239–89.
- ↑ Fuchs, A.; de Vries, F.W.; Platerno Sanz, M. (1980). "The mechanism of pisatin degradation by Fusarium oxysporum f. sp. pisi.". Physiol. Plant Pathol 16: 119–33. doi:10.1016/0048-4059(80)90025-9.
- ↑ Sanz Platero, de M.; Fuchs, A. (1978). "Degradation of pisatin, an antimicrobial compound produced by Pisum sativum L". Phytopathol. Mediterr. 17: 14–17.
- ↑ 10.0 10.1 10.2 Delserone, L.M.; VanEtten, H.D. (1987). "Demethylation of pisatin by three fungal pathogens of Pisum sativum". Phytopathology 77: 116 (Abstr.
- ↑ Wanner, L.A.; Ware, D.; Somssich, I.E.; Davis, K.R. (1995). "The phenylalanine ammonia-lyase gene family in Arabidopsis thaliana.". Plant Mol Biol 27 (2): 327–38. doi:10.1007/bf00020187. PMID 7888622.
- ↑ Mizutani, M.; Ohta, D.; Sato, R. (1997). "Isolation of a cDNA and a genomic clone encoding cinnamate 4-hydroxylase from Arabidopsis and its expression manner in planta.". Plant Physiol 113 (3): 755–63. doi:10.1104/pp.113.3.755. PMID 9085571.
- ↑ Nair, R.B.; Bastress, K.L.; Ruegger, M.O.; Denault, J.W.; Chapple, C. (2004). "The Arabidopsis thaliana reduced epidermal fluorescence 1 gene encodes an aldehyde dehydrogenase involved in ferulic acid and sinapic acid biosynthesis.". Plant Cell 16 (2): 544–54. doi:10.1105/tpc.017509. PMID 14729911.
- ↑ Joung, J.Y.; Kasthuri, G.M.; Park, J.Y.; Kang, W.J.; Kim, H.S.; Yoon, B.S.; Joung, H.; Jeon, J.H. (2003). "An overexpression of chalcone reductase of Pueraria montana var. lobata alters biosynthesis of anthocyanin and 5′-deoxyflavonoids in transgenic tobacco.". Biochem Biophys Res Commun 303 (1): 326–31. doi:10.1016/s0006-291x(03)00344-9. PMID 12646206.
- ↑ Kimura, Y.; Aoki, T.; Ayabe, S. (2001). "Chalcone isomerase isozymes with different substrate specificities towards 6′-hydroxy- and 6′-deoxychalcones in cultured cells of Glycyrrhiza echinata, a leguminous plant producing 5-deoxyflavonoids.". Plant Cell Physiol 42 (10): 1169–73. doi:10.1093/pcp/pce130. PMID 11673633.
- ↑ Kim, B.G.; Kim, S.Y.; Song, H.S.; Lee, C.; Hur, H.G.; Kim, S.I.; Ahn, J.H. (2003). "Cloning and expression of the isoflavone synthase gene (IFS-Tp) from Trifolium pratense.". Mol Cells 15 (3): 301–6. PMID 12872984.
- ↑ Pichersky, E.; Gang, D.R. (2000). "Genetics and biochemistry of secondary metabolites in plants: an evolutionary perspective.". Trends in Plant Science 5 (10): 439–445. doi:10.1016/s1360-1385(00)01741-6. PMID 11044721.
- ↑ Dewick, P.M.. "The flavonoids: Advances in research since 1986". Isoflavonoids (Chapman and Hall): 117–238.
- ↑ Wengenmayer, H.; Ebel, J.; Grisebach, H. (1974). "Purification and properties of a S-adenosylmethionine: isoflavone 4′-O-methyltransferase from cell suspension cultures of Cicer arietinum L.". Eur. J. Biochem. 50 (1): 135–143. doi:10.1111/j.1432-1033.1974.tb03881.x. PMID 4452353.
- ↑ Clemens, S.; Hinderer, W.; Wittkampg, U.; Barz, W. (1993). "Characterization of cytochrome P450-dependent isoflavone hydroxylase from chickpea.". Phytochemistry 32 (3): 653–657. doi:10.1016/s0031-9422(00)95150-1. Bibcode: 1993PChem..32..653C.
- ↑ Liu, C.J.; Huhman, D.; Sumner, L.W.; Dixon, R.A. (2003). "Regiospecific hydroxylation of isoflavones by cytochrome p450 81E enzymes from Medicago truncatula". Plant J 36 (4): 471–484. doi:10.1046/j.1365-313x.2003.01893.x. PMID 14617078. https://digital.library.unt.edu/ark:/67531/metadc488180/.
- ↑ Paiva; Sun, Y.; Dixon, R.A.; Van Etten, H.D.; Hrazdina, G. (1994). "Molecular cloning of isoflavone reductase from pea (Pisum sativum L.): evidence for a 3R-isoflavanone intermediate in (+)-pisatin biosynthesis.". Arch. Biochem. Biophys. 312 (2): 501–510. doi:10.1006/abbi.1994.1338. PMID 8037464.
- ↑ Bless, W.; Barz, W. (1988). "Isolation of pterocarpan synthase, the terminal enzyme of pterocarpan phytoalexin biosynthesis in cell suspension cultures of Cicer arietinum.". FEBS Letters 235 (1): 47–50. doi:10.1016/0014-5793(88)81231-6.
- ↑ Guo, N.; Dixon, R.A.; Paiva, N.L. (1994). "The pterocarpan synthase of alfalfa: association and co-induction of vestitone reductase and 7,2′-dihydroxy-4′-methoxy-isoflavanol (DMI) dehydratase, the two final enzymes in medicarpin biosynthesis.". FEBS Lett. 356 (2–3): 221–225. doi:10.1016/0014-5793(94)01267-9. PMID 7805842.
- ↑ Matthews, D.E.; Weiner, E.J.; Matthews, P.S.; VanEtten, H.D. (1987). "Role of oxygenases in pisatin biosynthesis and in the fungal degradation of maackiain". Plant Physiology 83 (2): 365–370. doi:10.1104/pp.83.2.365. PMID 16665251.
- ↑ Wu, Q.; Preisig, C.L.; VanEtten, H.D. (1997). "Isolation of the cDNAs encoding (+)6a-hydroxymaackiain 3-O-methyltransferase, the terminal step for the synthesis of the phytoalexin pisatin in Pisum satium.". Plant Mol. Biol. 35 (5): 551–560. doi:10.1023/A:1005836508844. PMID 9349277.
- ↑ Caspi (2014). "The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases". Nucleic Acids Research 42 (Database issue): D459–D471. doi:10.1093/nar/gkt1103. PMID 24225315.
 |
 KSF
KSF
