Poly(hydridocarbyne)
Topic: Chemistry
 From HandWiki - Reading time: 2 min
From HandWiki - Reading time: 2 min
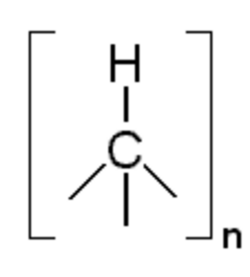
| |
| Identifiers | |
|---|---|
| ChemSpider |
|
| Properties | |
| [HC]n | |
| Molar mass | 200,000 to 100 million daltons |
| Melting point | decomposes @ 100 °C |
| Boiling point | N/A |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Poly(hydridocarbyne) (PHC) is one of a class of carbon-based random network polymers primarily composed of tetrahedrally hybridized carbon atoms, each having one hydride substituent, exhibiting the generic formula [HC]n. PHC is made from bromoform, a liquid halocarbon that is commercially manufactured from methane. At room temperature, poly(hydridocarbyne) is a dark brown powder. It can be easily dissolved in a number of solvents (tetrahydrofuran, ether, toluene etc.), forming a colloidal suspension that is clear and non-viscous, which may then be deposited as a film or coating on various substrates. Upon thermolysis in argon at atmospheric pressure and temperatures of 110 °C to 1000 °C, decomposition of poly(hydridocarbyne) results in hexagonal diamond (lonsdaleite).
More recently poly(hydridocarbyne) has been synthesized by a much simpler method using electrolysis of chloroform (May 2008)[1] and hexachloroethane (June 2009).[2]
The novelty of PHC (and its related polymer poly(methylsilyne)) is that the polymer may be readily fabricated into various forms (e.g. films, fibers, plates) and then thermolyzed into a final hexagonal diamond ceramic.
See also
- Carbyne, for name origin
References
- "Diamond and Diamond-like Carbon from a Preceramic Polymer". Journal of the American Chemical Society 126 (10): 3191–3202. 2004. doi:10.1021/ja039254l. PMID 15012149.
- "High molecular weight polymers". 2004. http://www.patentdebate.com/PATAPP/20040010108. (US patent application)
- "Facile Synthesis of Poly(hydridocarbyne): A Precursor to Diamond and Diamond-like Ceramics". Journal of Macromolecular Science, Part A 45 (5): 358–363. May 2008. doi:10.1080/10601320801946108.
- "Electrochemical polymerizatıon of hexachloroethane to form poly(hydridocarbyne): a pre-ceramic polymer for diamond production". Journal of Materials Science 44 (11): 2774–2779. June 2009. doi:10.1007/s10853-009-3364-4. Bibcode: 2009JMatS..44.2774N.
Notes
- ↑ Toppare L et al. (2008) Facile Synthesis of Poly(hydridocarbyne): A Precursor to Diamond and Diamond-like Ceramics, Journal of Macromolecular Science, Part A 45 (5), May, 358–363, doi=10.1080/10601320801946108.
- ↑ Toppare L et al. (2009) Electrochemical polymerizatıon of hexachloroethane to form poly(hydridocarbyne): a pre-ceramic polymer for diamond production, Journal of Materials Science 44 2774–2779, doi=10.1007/s10853-009-3364-4.
External links
- Facile Synthesis of Poly(hydridocarbyne): A Precursor to Diamond and Diamond-like Ceramics
- Liftport Staff Blog discussion
 |
 KSF
KSF