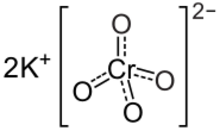
Potassium chromate
Topic: Chemistry
 From HandWiki - Reading time: 5 min
From HandWiki - Reading time: 5 min

| |
| |
| Names | |
|---|---|
| IUPAC name
Potassium chromate
| |
| Other names
Potassium dichromate, Chromic acid, (K2CrO4), dipotassium salt
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3077 |
| |
| |
| Properties | |
| K 2CrO 4 | |
| Molar mass | 194.189 g·mol−1 |
| Appearance | Yellow powder |
| Odor | odorless |
| Density | 2.7320 g/cm3 |
| Melting point | 968 °C (1,774 °F; 1,241 K) |
| Boiling point | 1,000 °C (1,830 °F; 1,270 K) |
| |
| 3.9×10−6 cm3/mol | |
Refractive index (nD)
|
1.74 |
| Structure | |
| rhombic | |
| Hazards | |
| Safety data sheet | Fisher Scientific[1] |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H315, H317, H319, H335, H340, H350, H410 | |
| P201, P202, P261, P264, P271, P272, P273, P280, P302+352, P304+340+312Script error: No such module "Preview warning".Category:GHS errors, P305+351+338, P308+313, P333+313, P337+313, P362, P391, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions
|
Potassium dichromate |
Other cations
|
|
Related chromates
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Potassium chromate is the inorganic compound with the formula K
2CrO
4. This yellow solid is the potassium salt of the chromate anion. It is a common laboratory chemical, whereas sodium chromate is important industrially.
Production and reactions
It is prepared by treating potassium dichromate with potassium hydroxide:
- K
2Cr
2O
7(aq) + 2 KOH → 2 K
2CrO
4 + H
2O
Or, the fusion of potassium hydroxide and chromium trioxide:
- 2 KOH + CrO
3 → K
2CrO
4 + H
2O
When treated with lead(II) nitrate, it gives an orange-yellow precipitate, lead(II) chromate.
Applications
Unlike the less expensive sodium salt, the potassium salt is mainly used for laboratory work in situations where an anhydrous salt is required, or as an oxidizing agent in organic synthesis.[3]
It is used in qualitative inorganic analysis, e.g. as a colorimetric test for silver ion. It is also used as an indicator in precipitation titrations with silver nitrate and sodium chloride (they can be used as standard as well as titrant for each other) as potassium chromate turns red in the presence of excess of silver ions.[citation needed]
Structure
Two crystalline forms are known, both being very similar to the corresponding potassium sulfate. Orthorhombic β-K
2CrO
4 is the common form, but it converts to an α-form above 666 °C (1,231 °F).[3] These structures are complex, although the chromate ion adopts the typical tetrahedral geometry.[4][better source needed]
-
Structure of β-K2CrO4
-
Coordination sphere of one of two types of K+
site -
The environment about the tetrahedral CrO2−
4 center in β-K
2CrO
4
Safety
As with other Cr(VI) compounds, potassium chromate is carcinogenic. Positive associations with lung cancer at a very high rate, and nasal / sinus cancer at a 100x lower rate have been found using worker exposure data. In general, less soluble chromates are a larger chronic hazard as they can be encapsulated in the lung without being absorbed and excreted, giving more time for reactive intermediates to be produced. Animal data indicates a potential for impaired fertility, heritable genetic damage and harm to unborn children, along with other types of cancer via less common exposure routes.[5]
As a highly soluble hexavalent chromium compound, potassium chromate is also acutely toxic, though it is poorly absorbed from the intestinal tract.[5] The compound is also corrosive and exposure may produce severe eye damage or blindness.[1]
References
- ↑ 1.0 1.1 1.2 "SDS - Potassium Chromate". Thermo Fisher Scientific. 29 March 2024. https://documents.thermofisher.com/DirectWebViewer/private/document.aspx?prd=ALFAA12610~~PDF~~MTR~~AGHS~~EN~~2024-03-30%2000:10:50~~Potassium%20chromate~~.
- ↑ Sigma-Aldrich Co., Potassium chromate.
- ↑ 3.0 3.1 Anger, Gerd; Halstenberg, Jost; Hochgeschwender, Klaus; Ulrich Korallus, Christoph Scherhag; Knopf, Herbert; Schmidt, Peter; Ohlinger, Manfred. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a07_067.
- ↑ Gaultier, M.; Pannetier, G. "Structure cristalline de la forme 'basse temperature' du sulfate de potassium K2SO4-beta" (Crystal structure of the "low temperature" β-form of potassium sulfate) Bulletin de la Société Chimique de France 1968, vol. 1, pp. 105-12.
- ↑ 5.0 5.1 Volume 100C: Arsenic, Metals, Fibres, and Dusts. Lyon, France: World Health Organization - International Agency for Research on Cancer. 2012. pp. 153-64. ISBN 978-92-832-0135-9. https://publications.iarc.who.int/_publications/media/download/6143/ef2dcba35d394362f6f5346d042bd48e5792ded3.pdf. Retrieved 2020-01-05.
Template:Chromates and dichromates
 |
 KSF
KSF
