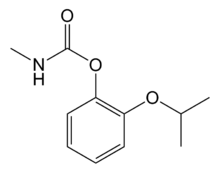
Propoxur
Topic: Chemistry
 From HandWiki - Reading time: 4 min
From HandWiki - Reading time: 4 min
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-[(Propan-2-yl)oxy]phenyl methylcarbamate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C11H15NO3 | |
| Molar mass | 209.245 g·mol−1 |
| Appearance | White to tan crystalline powder[1] |
| Odor | faint, characteristic[1] |
| Melting point | 86 to 92 °C; 187 to 197 °F; 359 to 365 K |
| Boiling point | decomposes[1] |
| 0.2% (20°C)[1] | |
| Vapor pressure | 0.0000937 mmHg (20 °C)[1] |
| Pharmacology | |
| 1=ATCvet code} | QP53AE02 (WHO) |
| Hazards | |
| Flash point | > 149 °C; 300 °F; 422 K |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
none[1] |
REL (Recommended)
|
TWA 0.5 mg/m3[1] |
IDLH (Immediate danger)
|
N.D.[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Propoxur (Baygon) is a carbamate non-systemic insecticide, produced from catechol,[2] and was introduced in 1959. It has a fast knockdown and long residual effect, and is used against turf, forestry, and household pests and fleas. It is also used in pest control for domestic animals, Anopheles mosquitoes, ants, gypsy moths, and other agricultural pests.[3][4] It can also be used as a molluscicide.[4][5][6]
Several U.S. states have petitioned the Environmental Protection Agency (EPA) to use propoxur against bedbug infestations, but the EPA has been reluctant to approve indoor use because of its potential toxicity to children after chronic exposure.[7]
Action
Carbamate insecticides kill insects by irreversibly inactivating the enzyme acetylcholinesterase, thus it is a Cholinesterase inhibitor.
Environmental effects
Propoxur rapidly breaks down in alkaline solution.[8] Propoxur is highly toxic to many bird species, although its toxicity varies by the species, and it is highly toxic to honeybees.[6] It is moderately to slightly toxic to fish and other aquatic species.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 NIOSH Pocket Guide to Chemical Hazards. "#0531". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0531.html.
- ↑ Fiege, Helmut; Voges, Heinz-Werner; Hamamoto, Toshikazu; Umemura, Sumio; Iwata, Tadao; Miki, Hisaya; Fujita, Yasuhiro; Buysch, Hans-Josef et al. (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_313.
- ↑ ACGIH, 1991a[full citation needed]
- ↑ 4.0 4.1 Budavari, 1996a[full citation needed]
- ↑ Lewis, 1993a[full citation needed]
- ↑ 6.0 6.1 EXTOXNET Extension Toxicology Network. Pesticide Information Profile. Propoxur. June 1996.
- ↑ "In Search of a Bedbug Solution". New York Times. September 4, 2010. https://www.nytimes.com/2010/09/05/opinion/05sun3.html.
- ↑ Propoxur (WHO Pesticide Residues Series 3): October 01, 2009.
 |
 KSF
KSF