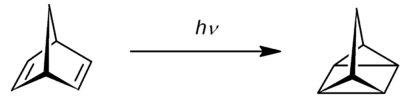Quadricyclane
Topic: Chemistry
 From HandWiki - Reading time: 3 min
From HandWiki - Reading time: 3 min

| |
| Names | |
|---|---|
| Preferred IUPAC name
Tetracyclo[3.2.0.02,7.04,6]heptane | |
| Other names
quadricyclo[2.2.1.02,6.03.5]heptane, tetracyclo[2.2.1.02,6.03.5]heptane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H8 | |
| Molar mass | 92.14 g/mol |
| Density | 0.982 g/cm3 |
| Melting point | −44 °C (−47 °F; 229 K) |
| Boiling point | 108 °C (226 °F; 381 K) at 987 hPa |
| Insoluble | |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H226, H330 | |
| P210, P260, P284, P310 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Quadricyclane is a strained, multi-cyclic hydrocarbon with the formula CH2(CH)6. A white volatile colorless liquid, it is highly strained molecule (78.7 kcal/mol). Isomerization of quadricyclane proceeds slowly at low temperatures.[1] Because of quadricyclane's strained structure and thermal stability, it has been studied extensively.
Preparation
Quadricyclane is produced by the irradiation of norbornadiene (bicyclo[2.2.1]hepta-2,5-diene)[2] in the presence of Michler's ketone or ethyl Michler's ketone.[3] Other sensitizers, such as acetone, benzophenone, acetophenone, etc., may be used but with a lesser yield. The yield is higher for freshly distilled norbornadiene, but commercial reagents will suffice.[2]
Proposed applications to solar energy
The conversion of norbornadiene into quadricyclane is achieved with ~300 nm UV radiation.[4] When converted back to norbornadiene, ring strain energy is liberated in the form of heat (ΔH = −89 kJ/mol). This reaction has been proposed to store solar energy.[5][6] However, the absorption edge of light does not extend past 300 nm whereas most solar radiation has wavelengths longer than 400 nm. Quadricyclane's relative stability and high energy content have also given rise to its use as a propellant additive or fuel. However, quadricyclane undergoes thermal decomposition at relatively low temperatures (less than 400 °C). This property limits its applications, as propulsion systems may operate at temperatures exceeding 500 °C.[7]
Reactions
Quadricyclane readily reacts with acetic acid to give a mixture of nortricyclyl acetate and exo-norbornyl acetate.[1] Quadricyclane also reacts with many dienophiles to form 1:1 adducts.[2]
Notes
- ↑ 1.0 1.1 Petrov, V. A; Vasil’ev, N. V. “Synthetic Chemistry of Quadricyclane.” Current Organic Synthesis 3 (2006): 215–259
- ↑ 2.0 2.1 2.2 Smith, Claiborune D. (1971). "Quadricyclane". Organic Syntheses. doi:10.15227/orgsyn.051.0133.
- ↑ Cahill, P; Steppel, R. Process of quadricyclane production. U.S. Patent 10,661,194 filed September 12, 2003, and issued March 18, 2004
- ↑ Kalsi, P S (2000). Organic Reactions And Their Mechanisms. New Age International. p. 366. ISBN 978-81-224-1268-0. https://books.google.com/books?id=Zr7algbSigoC&pg=PA366.
- ↑ Dubonosov, A. D; Bren, V. A; Chernoivanov, V. A. “Norbornadiene – quadricyclane as an abiotic system for the storage of solar energy.” Russian Chemical Reviews 71 (2002): 917–927
- ↑ Philippopoulos, Constantine; Economou, Dimitrios; Economou, Constantine; Marangozis, John (1983). "Norbornadiene-quadricyclane system in the photochemical conversion and storage of solar energy". Industrial & Engineering Chemistry Product Research and Development 22 (4): 627. doi:10.1021/i300012a021.
- ↑ Striebich, R; Lawrence, J (2003). "Thermal decomposition of high-energy density materials at high pressure and temperature". Journal of Analytical and Applied Pyrolysis 70 (2): 339. doi:10.1016/S0165-2370(02)00181-X.
 |
 KSF
KSF