Quinoline alkaloids
Topic: Chemistry
 From HandWiki - Reading time: 1 min
From HandWiki - Reading time: 1 min
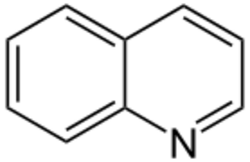
Quinoline alkaloids are naturally occurring chemical compounds from the group of alkaloids, which are chemically derived from quinoline. Some quinoline alkaloids show antiseptic, convulsive or antineoplastic effects.
Examples
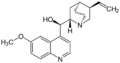
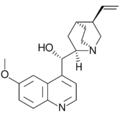
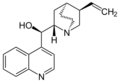
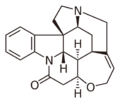
Alkaloids with a quinoline partial structure are widespread and are usually further subdivided according to their occurrence and biogenetic origin. Among the quinoline alkaloids are the cinchona alkaloids quinine and quinidine, which are important due to their therapeutic potential, furthermore cinchonine and cinchonidine, as well as some furoquinoline alkaloids and acridine alkaloids. Strychnine and brucine, alkaloids of the nux vomica, which have a hydrogenated quinoline system, are also counted among the quinoline alkaloids. Also nitramarine (1-(2-quinolinyl)-β-carboline) belongs to the quinoline alkaloids.
Occurrence
The quinoline alkaloids are mainly found in plants, as in Rutaceae and Rubiaceae, but also in microorganisms and animals. Quinoline is furthermore comprised as a partial structure in the redox factor PQQ (pyrroloquinoline quinone) and in quinoenzymes.
Biosynthesis
Biogenetically, there are several proven pathways for the formation of the quinoline system in plants. Tryptophane as well as anthranilic acid can act as a precursor. The second precursor molecule is either a hemiterpene or a monoterpene (e.g. secologanine in cinchona alkaloids).
Literature
- Entry on Chinolin-Alkaloide. at: Römpp Online. Georg Thieme Verlag, retrieved 26. Juni 2014.
This article does not cite any external source. HandWiki requires at least one external source. See citing external sources. (2021) (Learn how and when to remove this template message) |
 |
 KSF
KSF