Reuptake inhibitor
Topic: Chemistry
 From HandWiki - Reading time: 8 min
From HandWiki - Reading time: 8 min
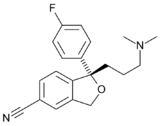
Reuptake inhibitors (RIs) are a type of reuptake modulators. It is a drug that inhibits the plasmalemmal transporter-mediated reuptake of a neurotransmitter from the synapse into the pre-synaptic neuron. This leads to an increase in extracellular concentrations of the neurotransmitter and an increase in neurotransmission. Various drugs exert their psychological and physiological effects through reuptake inhibition, including many antidepressants and psychostimulants.[1]
Most known reuptake inhibitors affect the monoamine neurotransmitters serotonin, norepinephrine (and epinephrine), and dopamine.[1] However, there are also a number of pharmaceuticals and research chemicals that act as reuptake inhibitors for other neurotransmitters such as glutamate,[2] γ-aminobutyric acid (GABA),[3] glycine,[4] adenosine,[5] choline (the precursor of acetylcholine),[6] and the endocannabinoids,[7] among others.[1]
Mechanism of action
This section's factual accuracy is disputed. (January 2016) (Learn how and when to remove this template message) |
Active site transporter substrates
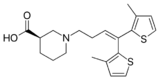
Standard reuptake inhibitors are believed to act simply as competitive substrates that work by binding directly to the plasmalemma transporter of the neurotransmitter in question.[8][9][10][11] They occupy the transporter in place of the respective neurotransmitter and competitively block it from being transported from the nerve terminal or synapse into the pre-synaptic neuron. With high enough doses, occupation becomes as much as 80–90%. At this level of inhibition, the transporter will be considerably less efficient at removing excess neurotransmitter from the synapse and this causes a substantial increase in the extracellular concentrations of the neurotransmitter and therefore an increase in overall neurotransmission.
Allosteric site transporter substrates
Alternatively, some reuptake inhibitors bind to allosteric sites and inhibit reuptake indirectly and noncompetitively.
Phencyclidine and related drugs such as benocyclidine, tenocyclidine, ketamine, and dizocilpine (MK-801), have been shown to inhibit the reuptake of the monoamine neurotransmitters.[12][13][14] They appear to exert their reuptake inhibition by binding to vaguely characterized allosteric sites on each of the respective monoamine transporters.[15][16][17][18][19] Benztropine, mazindol, and vanoxerine also bind to these sites and have similar properties.[15][19][20] In addition to their high affinity for the main site of the monoamine transporters, several competitive transporter substrates such as cocaine and indatraline have lower affinity for these allosteric sites as well.[17][19][20]
A few of the selective serotonin reuptake inhibitors (SSRIs) such as the dextro-enantiomer of citalopram appear to be allosteric reuptake inhibitors of serotonin.[21][22] Instead of binding to the active site on the serotonin transporter, they bind to an allosteric site, which exerts its effects by causing conformational changes in the transporter protein and thereby modulating the affinity of substrates for the active site.[21] As a result, escitalopram has been marketed as an allosteric serotonin reuptake inhibitor. Notably, this allosteric site may be directly related to the above-mentioned PCP binding sites.[15][20]
Vesicular transporter substrates
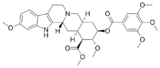
A second type of reuptake inhibition affects vesicular transport, and blocks the intracellular repackaging of neurotransmitters into cytoplasmic vesicles. In contrast to plasmalemmal reuptake inhibitors, vesicular reuptake inhibitors do not increase the synaptic concentrations of a neurotransmitter, only the cytoplasmic concentrations; unless, that is, they also act as plasmalemmal transporter reversers via phosphorylation of the transporter protein, also known as a releasing agent. Pure vesicular reuptake inhibitors tend to actually lower synaptic neurotransmitter concentrations, as blocking the repackaging of, and storage of the neurotransmitter in question leaves them vulnerable to degradation via enzymes such as monoamine oxidase (MAO) that exist in the cytoplasm. With vesicular transport blocked, neurotransmitter stores quickly become depleted.
Reserpine (Serpasil) is an irreversible inhibitor of the vesicular monoamine transporter 2 (VMAT2), and is a prototypical example of a vesicular reuptake inhibitor.
Indirect unknown mechanism
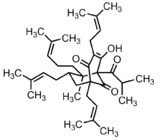
Two of the primary active constituents of the medicinal herb Hypericum perforatum (St. John's Wort) are hyperforin and adhyperforin.[23][24] Hyperforin and adhyperforin are wide-spectrum inhibitors of the reuptake of serotonin, norepinephrine, dopamine, glutamate, GABA, glycine,[25] and choline,[26] and they exert these effects by binding to and activating the transient receptor potential cation channel TRPC6.[24][27] Activation of TRPC6 induces the entry of calcium (Ca2+) and sodium (Na+) into the cell, which causes the effect through unknown mechanism.[27]
Types
Typical
- Amino acid reuptake inhibitor
- Excitatory amino acid reuptake inhibitor (or glutamate-aspartate reuptake inhibitor)
- GABA reuptake inhibitor
- Glycine reuptake inhibitor
- Monoamine reuptake inhibitor
- Dopamine reuptake inhibitor
- Norepinephrine reuptake inhibitor
- Serotonin reuptake inhibitor
- Serotonin-norepinephrine reuptake inhibitor
- Norepinephrine-dopamine reuptake inhibitor
- Serotonin-dopamine reuptake inhibitor
- Serotonin-norepinephrine-dopamine reuptake inhibitor
- Miscellaneous
- Adenosine reuptake inhibitor
- Endocannabinoid reuptake inhibitor
Atypical
- TRPC6 activators (wide-spectrum reuptake inhibitors) – hyperforin, adhyperforin
Plasmalemmal
- Choline reuptake inhibitor – hemicholinium-3, triethylcholine
Vesicular
- Vesicular acetylcholine transporter (VAChT) inhibitor – vesamicol
- Vesicular monoamine transporter (VMAT) inhibitor – reserpine, tetrabenazine
See also
- Releasing agent
References
- ↑ 1.0 1.1 1.2 Iversen L. (2006). "Neurotransmitter transporters and their impact on the development of psychopharmacology.". Br J Pharmacol 147 (1): S82–88. doi:10.1038/sj.bjp.0706428. PMID 16402124.
- ↑ "Inhibition of glutamate reuptake potentiates endogenous nitric oxide-facilitated dopamine efflux in the rat striatum: an in vivo microdialysis study". Neurosci. Lett. 230 (1): 21–4. 1997. doi:10.1016/S0304-3940(97)00465-5. PMID 9259454.
- ↑ "The selective GABA reuptake inhibitor tiagabine for the treatment of generalized anxiety disorder: results of a placebo-controlled study". J Clin Psychiatry 66 (11): 1401–8. 2005. doi:10.4088/JCP.v66n1109. PMID 16420077.
- ↑ "Glycine reuptake inhibitor RG1678: a pharmacologic characterization of an investigational agent for the treatment of schizophrenia". Neuropharmacology 62 (2): 1152–61. February 2012. doi:10.1016/j.neuropharm.2011.11.008. PMID 22138164.
- ↑ "The effects of the adenosine reuptake inhibitor soluflazine on synaptic potentials and population hypoxic depolarizations in area CA1 of rat hippocampus in vitro". Neuropharmacology 32 (2): 149–55. 1993. doi:10.1016/0028-3908(93)90095-K. PMID 8383814.
- ↑ "Evaluation of the inhibition of choline uptake in synaptosomes by capillary electrophoresis with electrochemical detection". Electrophoresis 23 (21): 3699–704. November 2002. doi:10.1002/1522-2683(200211)23:21<3699::AID-ELPS3699>3.0.CO;2-E. PMID 12432531.
- ↑ "AM404, an inhibitor of anandamide uptake, prevents pain behaviour and modulates cytokine and apoptotic pathways in a rat model of neuropathic pain". Br J Pharmacol 148 (7): 1022–32. 2006. doi:10.1038/sj.bjp.0706798. PMID 16770320.
- ↑ Barker, Eric L.; Randy D. Blakely (1995). Norepinephrine and serotonin transporters: molecular targets of antidepressant drugs. In: Psychopharmacology: the fourth generation of progress..
- ↑ "Distinct effects of imipramine on 5-hydroxytryptamine uptake mediated by the recombinant rat serotonin transporter SERT1.". Journal of Neurochemistry 70 (6): 2545–2553. 1998. doi:10.1046/j.1471-4159.1998.70062545.x. PMID 9603221.
- ↑ "Molecular mechanism of citalopram and cocaine interactions with neurotransmitter transporters.". J Pharmacol Exp Ther 307 (1): 34–41. 2003. doi:10.1124/jpet.103.054593. PMID 12944499.
- ↑ "Antidepressants targeting the serotonin reuptake transporter act via a competitive mechanism.". J Pharmacol Exp Ther 327 (3): 982–990. 2008. doi:10.1124/jpet.108.142315. PMID 18801947.
- ↑ "The role of antagonism of NMDA receptor-mediated neurotransmission and inhibition of the dopamine reuptake in the neuroendocrine effects of phencyclidine". Life Sci. 78 (17): 2006–11. 2006. doi:10.1016/j.lfs.2005.09.018. PMID 16288927.
- ↑ "Ketamine inhibits monoamine transporters expressed in human embryonic kidney 293 cells". Anesthesiology 88 (3): 768–74. 1998. doi:10.1097/00000542-199803000-00029. PMID 9523822.
- ↑ "MK-801 blocks monoamine transporters expressed in HEK cells.". FEBS Lett. 423 (3): 376–380. 1998. doi:10.1016/S0014-5793(98)00126-4. PMID 9515743.
- ↑ 15.0 15.1 15.2 "[3H1-[2-(2-thienyl)cyclohexyl]piperidine labels two high-affinity binding sites in human cortex: further evidence for phencyclidine binding sites associated with the biogenic amine reuptake complex."]. Synapse 8 (4): 289–300. 1991. doi:10.1002/syn.890080407. PMID 1833849. https://zenodo.org/record/1229376.
- ↑ "The psychotomimetic drug phencyclidine labels two high affinity binding sites in guinea pig brain: evidence for N-methyl-D-aspartate-coupled and dopamine reuptake carrier-associated phencyclidine binding sites.". Mol. Pharmacol. 36 (6): 887–896. 1989. PMID 2557536.
- ↑ 17.0 17.1 "RTI-4793-14, a new ligand with high affinity and selectivity for the (+)-MK801-insensitive [3H1-]1-(2-thienyl)cyclohexyl]piperidine binding site (PCP site 2) of guinea pig brain."]. Synapse 16 (1): 59–65. 1994. doi:10.1002/syn.890160107. PMID 8134901. https://zenodo.org/record/1229378.
- ↑ Rothman RB. (1994). "PCP site 2: a high affinity MK-801-insensitive phencyclidine binding site.". Neurotoxicol Teratol 16 (4): 343–353. doi:10.1016/0892-0362(94)90022-1. PMID 7968938. https://zenodo.org/record/1258623.
- ↑ 19.0 19.1 19.2 "Studies of the biogenic amine transporters. VI. Characterization of a novel cocaine binding site, identified with [125I]RTI-55, in membranes prepared from whole rat brain minus caudate.". J Pharmacol Exp Ther 274 (1): 385–395. 1995. PMID 7616423.
- ↑ 20.0 20.1 20.2 "Studies of the biogenic amine transporters. IV. Demonstration of a multiplicity of binding sites in rat caudate membranes for the cocaine analog [125I]RTI-55.". J Pharmacol Exp Ther 270 (1): 296–309. 1994. PMID 8035327.
- ↑ 21.0 21.1 "The S-enantiomer of R,S-citalopram, increases inhibitor binding to the human serotonin transporter by an allosteric mechanism. Comparison with other serotonin transporter inhibitors.". Eur. Neuropsychopharmacol. 15 (2): 193–198. 2005. doi:10.1016/j.euroneuro.2004.08.008. PMID 15695064.
- ↑ "Allosteric modulation of the effect of escitalopram, paroxetine and fluoxetine: in-vitro and in-vivo studies.". Int J Neuropsychopharmacol 10 (1): 31–40. 2007. doi:10.1017/S1461145705006462. PMID 16448580.
- ↑ "Hyperforin – antidepressant activity by a novel mechanism of action". Pharmacopsychiatry 34 (Suppl 1): S98–102. 2001. doi:10.1055/s-2001-15512. PMID 11518085.
- ↑ 24.0 24.1 "Hyperforin as a possible antidepressant component of hypericum extracts". Life Sci. 63 (6): 499–510. 1998. doi:10.1016/S0024-3205(98)00299-9. PMID 9718074.
- ↑ "The involvement of sodium and calcium ions in the release of amino acid neurotransmitters from mouse cortical slices elicited by hyperforin". Life Sciences 71 (22): 2645–55. October 2002. doi:10.1016/S0024-3205(02)02104-5. PMID 12354583.
- ↑ "Dual modulation of striatal acetylcholine release by hyperforin, a constituent of St. John's wort". The Journal of Pharmacology and Experimental Therapeutics 301 (2): 714–9. May 2002. doi:10.1124/jpet.301.2.714. PMID 11961077.
- ↑ 27.0 27.1 "Hyperforin – a key constituent of St. John's wort specifically activates TRPC6 channels". The FASEB Journal 21 (14): 4101–11. December 2007. doi:10.1096/fj.07-8110com. PMID 17666455.
 |
 KSF
KSF