Rosenmund reduction
Topic: Chemistry
 From HandWiki - Reading time: 2 min
From HandWiki - Reading time: 2 min
| Rosenmund reduction | |
|---|---|
| Named after | Karl Wilhelm Rosenmund |
| Reaction type | Organic redox reaction |
| Identifiers | |
| Organic Chemistry Portal | rosenmund-reduction |
| RSC ontology ID | RXNO:0000136 |
The Rosenmund reduction is a hydrogenation process in which an acyl chloride is selectively reduced to an aldehyde. The reaction was named after Karl Wilhelm Rosenmund, who first reported it in 1918.[1]
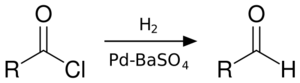
The reaction, a hydrogenolysis, is catalysed by palladium on barium sulfate, which is sometimes called the Rosenmund catalyst. Barium sulfate has a low surface area which reduces the activity of the palladium, preventing over-reduction. However, for certain reactive acyl chlorides the activity must be reduced further, by the addition of a poison. Originally this was thioquinanthrene although thiourea[2] has also been used.[3][4] Deactivation is required because the system must reduce the acyl chloride but not the subsequent aldehyde. If further reduction does take place, it will create a primary alcohol which would then react with the remaining acyl chloride to form an ester.
Rosenmund catalyst can be prepared by reduction of palladium(II) chloride solution in the presence of BaSO4. Typical reducing agent is formaldehyde.[5]
While Rosenmund reduction method can be used to prepare several aldehydes, formaldehyde cannot be prepared, as formyl chloride is unstable at room temperatures.[6]

See also
- Lindlar catalyst - Palladium on calcium carbonate with added catalytic poison, similar to the Rosenmund catalyst
- Grundmann aldehyde synthesis - Also reduces an acyl halide to an aldehyde, via the use of diazomethane
- Diisobutylaluminium hydride (DIBALH) can also reduce acid chlorides to aldehydes.
- Stephen aldehyde synthesis - Nitriles to aldehydes via reduction
References
- ↑ Rosenmund, K. W. (1918). "Über eine neue Methode zur Darstellung von Aldehyden. 1. Mitteilung" (in de). Chemische Berichte 51: 585–593. doi:10.1002/cber.19180510170. https://zenodo.org/record/1426637.
- ↑ Weygand, Conrad; Meusel, Werner (12 May 1943). "Über die Abstimmung der katalytischen Hydrierung, III. Mitteil.: Thioharnstoff als Spezifikator bei der Bildung von Benzaldehyd aus Benzoylchlorid" (in de). Berichte der Deutschen Chemischen Gesellschaft (A and B Series) 76 (5): 503–504. doi:10.1002/cber.19430760510.
- ↑ Rosenmund, K. W.; Zetzsche, F. (1921). "Über die Beeinflussung der Wirksamkeit von Katalysatoren, 1. bis 5" (in de). Chemische Berichte 54 (3): 425–437; 638–647; 1092–1098; 2033–2037; 2038–2042. doi:10.1002/cber.19210540310. https://zenodo.org/record/1426687.
- ↑ Mosettig, E.; Mozingo, R. (1948). "The Rosenmund Reduction of Acid Chlorides to Aldehydes". Organic Reactions 4: 362–377. doi:10.1002/0471264180.or004.07. ISBN 0471264180.
- ↑ Mozingo, Ralph (1946). "Palladium catalysts". Organic Syntheses 26: 77–82. doi:10.15227/orgsyn.026.0077. PMID 20280763.
- ↑ Srivastava, H.C.. Nootan ISC Chemistry Class XII. pp. 691. ISBN 978-9383877034.
Further reading
- Rachlin, A. I.; Gurien, H.; Wagner, D. P. (1988). "Aldehydes from Acid Chlorides by modified Rosenmund Reduction: 3,4,5-Trimethoxybenzaldehyde". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv6p1007.; Collective Volume, 6, pp. 1007
- Saytzeff, M. (1873). "Ueber die Einwirkung des vom Palladium absorbirten Wasserstoffes auf einige organische Verbindungen". Journal für Praktische Chemie 6 (1): 128–135. doi:10.1002/prac.18730060111. https://zenodo.org/record/1427856.
 |
 KSF
KSF