Selective androgen receptor modulator
Topic: Chemistry
 From HandWiki - Reading time: 24 min
From HandWiki - Reading time: 24 min
| Selective androgen receptor modulator | |
|---|---|
| Drug class | |
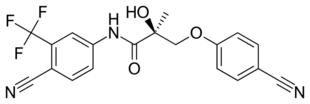 Enobosarm (ostarine), a nonsteroidal SARM under investigation for potential medical use. | |
| Class identifiers | |
| Synonyms | Nonsteroidal androgen (although not all SARMs are nonsteroidal)[1] |
| Use | Investigational |
| Biological target | Androgen receptor |
| Chemical class | Mostly nonsteroidal |
Selective androgen receptor modulators (SARMs) are a class of drugs that selectively activate the androgen receptor in certain tissues like muscle and bone over other tissues like the prostate gland and seminal vesicles.
Non-selective anabolic androgenic steroids (AAS) are potentially useful for a variety of medical conditions, but their use is limited by side effects. Attempts to find a steroid with anabolic effects in skeletal muscle and bone—increasing bone density and lean body mass—and negligible activity in other tissues were a failure. In 1998, researchers discovered a new class of nonsteroidal compounds (the SARMs) that selectively bind to the androgen receptor, granting them potent actions in muscle and bone with much less effect in reproductive tissues like the prostate gland and seminal vesicles.
SARMs have been investigated in human studies for the treatment of osteoporosis, cachexia (wasting syndrome), benign prostatic hyperplasia, stress urinary incontinence, and breast cancer. As of 2020[update], there are no SARMs which have been approved by the United States Food and Drug Administration. Although adverse effects in clinical studies have been infrequent and mild, SARMs can cause elevated liver enzymes, reduction of HDL cholesterol levels, and hypothalamic–pituitary–gonadal axis (HPG axis) suppression, among other side effects.
Since the early twenty-first century, SARMs have been used in doping; they were banned by the World Anti-Doping Agency in 2008. SARMs are readily available on internet-based gray markets and are commonly used recreationally to stimulate muscle growth.
History
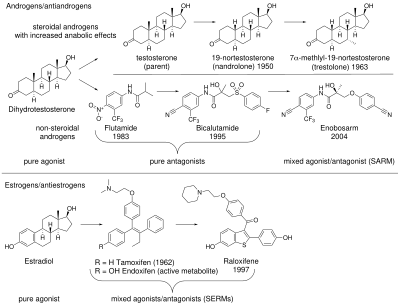
Anabolic androgenic steroids (AAS), including those produced endogenously such as testosterone and dihydrotestosterone (DHT), bind to and activate the androgen receptor (AR) to produce their effects. AAS effects can be separated into androgenic (the development and maintenance of male sexual characteristics) and anabolic (increasing bone density, muscle mass and strength). AAS also affect hematopoiesis, coagulation, metabolism, and cognition.[4][5] In the 1930s, 17α-alkylated anabolic steroids were discovered, which are sometimes considered SARMs due to greater tissue selectivity than testosterone.[6][7][8] These steroids were formed by adding an alkyl group to the testosterone molecule, changing its binding affinity to the AR.[9] 17α-Alkylated anabolic steroids still have significant androgenic effects, and are also hepatotoxic.[7] Efforts to develop a steroid with anabolic but minimal androgenic effects were not successful.[10]
Antiandrogens such as bicalutamide, flutamide, and nilutamide are nonsteroidal AR antagonists that work by binding to the AR to prevent androgenic action; this class of chemicals dates to the 1970s.[4][11] Interest in nonsteroidal AR agonists increased after the therapeutic uses of selective estrogen receptor modulators (SERMs) became evident.[11] The discovery of arylpropionamides, which share structural similarity with bicalutamide and hydroxyflutamide, suggested a way to make compounds that attach to the AR and produce both anabolic and antiandrogenic effects.[4] Selective androgen receptor modulators (SARMs) were developed out of a desire to maintain the anabolic effects of androgens on muscle and bone, while avoiding side effects on other tissues such as the prostate and cardiovascular system.[9]
Nonsteroidal SARMs were invented in 1998 independently by two research groups, one at the University of Tennessee that created an arylpropionamide SARM and Ligand Pharmaceuticals that made a SARM with a quinolone. The name was adopted by analogy with SERMs.[11] Other SARMs include tetrahydroquinolines, tricyclics, bridged tricyclics, aniline, diaryl aniline, bicylclic hydantoins, benzimidazole, imidazolopyrazole, indole, and pyrazoline derivatives.[4] SARMs can be agonists, antagonists, or partial agonists of the AR depending on the tissue, which can enable targeting specific medical conditions while minimizing side effects.[5] Those that have advanced to human trials show stronger effects in bone and muscle tissue and weaker effects in the prostate.[6] SARMs are orally bioavailable and largely eliminated via hepatic metabolism and metabolized through amide hydrolysis and A-ring nitro reduction.[9]
Mechanism
The mechanism of action of SARMs' tissue-specific effects continues to be debated as of 2020[update].[4][12] A number of hypotheses have been advanced. These include the non-activation of SARMs by 5α-reductase, tissue selective expression of androgen receptor coregulators, tissue selective uptake of SARMs, and non-genomic signaling.[4][13]
5α-Reductase
Testosterone is active in non-reproductive tissue without activation. In contrast, tissue selective activation by 5α-reductase to the more active form DHT is required for significant activity in reproductive tissue. The net result is that testosterone and its metabolite together are not tissue selective.[14] SARMs are not substrates of 5α-reductase, hence they are not selectively activated like testosterone in tissues such as prostate.[7] This lack of activation effectively imparts a degree of tissue selectivity to SARMS.
Androgen receptor coregulators
Tissue selective transcription coregulator expression is another possible contributor to the selectivity of SARMs.[15][13] Like other type I nuclear receptors, the uncomplexed androgen receptor (AR) resides in the cytosol. Upon ligand binding, the AR is translocated into the nucleus where it binds to androgen response elements on DNA to regulate gene expression.[16] AR agonists such as testosterone recruit coactivator proteins to AR that facilitate upregulation of gene expression while antagonists recruit corepressors which down regulate gene expression. Furthermore, the ratio of coactivators to corepressors is known to vary depending on tissue type.[15][17] Structurally, pure AR agonists stabilize the position of helix-12 (H12) in the ligand binding domain of AR near H3 and H4 to produce a surface cleft that binds to a FxxLF motif contained in coactivators.[16] Conversely, antagonists destabilize the agonist conformation of H12 blocking the binding of the FXXLF coactivator motif while facilitating the binding of the corepressor LXX(I/H)IXXX(I/L) motif found in NCOR1 and SMRT corepressors.[16]
In analogy to SERMs, SARMs are mixed agonists/antagonists displaying agonist androgen receptor activity in bone and muscle and partial agonist or antagonist activity in other tissues such as prostate.[13][5] Non-selective agonists such as testosterone are able to recruit coactivators when bound to AR but not corepressors and hence are agonists in all tissues. In contrast, SARMs can recruit both coactivators and corepressors by partially destabilizing the agonist conformation of H12. In tissues where coactivators are in excess (as in bone and muscle), SARMs act as agonists. Conversely, in tissues where corepressors are in excess (such as prostate), SARMs act as partial agonists or antagonists.[13]
In vitro testing of the SARMs enobosarm (ostarine) and YK-11 showed that they bound to the AR, but unlike full AR agonists, they blocked interaction between the N-terminus and C-terminus of AR which resulted in a mixed agonist/antagonist mode of action.[4][13]
Tissue distribution
Tissue selective uptake into anabolic tissues presents another potential mechanism for SARM tissue selectivity. However autoradiography studies with radiolabeled SARMs show no preferential distribution to anabolic tissues.[7]
Drug candidates
| Name | Class | Developer | Investigated for | Highest development stage reached | Structure |
|---|---|---|---|---|---|
| Andarine (S-4, GTx-007) | Arylpropionamide | GTx, Oncternal Therapeutics[19] | Cachexia[19] | Phase I (discontinued)[19] | 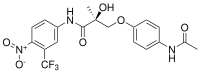
|
| Arcarine (ORM-11984)[20] | Unknown[18] | Orion Corporation[20] | Benign prostatic hyperplasia, hypogonadism, osteoporosis[18] | Phase I (discontinued)[20][18] | Structure undisclosed[18] |
| Enobosarm (ostarine, GTx-024, MK-2866, S-22) | Arylpropionamide | GTx, Veru Healthcare[21] | Breast cancer, cachexia, muscular dystrophy, stress urinary incontinence[21] | Phase III[21] | 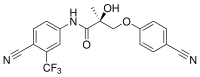
|
| DT-200 (GLPG-0492) | Imidazolidine-2,4-dione | ProSkelia, Akashi Therapeutics, Galapagos NV[22] | Muscular dystrophy, cachexia[22] | Phase I[18][22] | Error creating thumbnail: Unable to save thumbnail to destination |
| GSK-971086 | Unknown[23] | GlaxoSmithKline[23] | Sarcopenia[23] | Phase I (discontinued)[23] | Structure undisclosed[23] |
| GSK-2849466 | Unknown[24] | GlaxoSmithKline[24] | Cachexia, heart failure[24] | Phase I (discontinued)[24] | Structure undisclosed[24] |
| GSK-2881078 | Indole | GlaxoSmithKline[25] | Cachexia[25][26] | Phase II[25] | 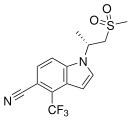
|
| LGD-2941 (LGD-122941) | Quinolinone | Ligand Pharmaceuticals[27] | Cachexia, sexual dysfunction, hypogonadism, menopause, osteoporosis[27] | Phase I (discontinued)[27] | Error creating thumbnail: Unable to save thumbnail to destination |
| LGD-4033 (VK5211, ligandrol) | Pyrrolidinebenzonitrile | Ligand Pharmaceuticals[28] | Muscle wasting due to hip fracture, cachexia, hypogonadism, osteoporosis[12][28] | Phase II[28] | 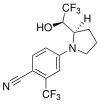
|
| LY305 | N-arylhydroxyalkyl | Eli Lilly[29] | Osteoporosis[29] | Phase I[29] | 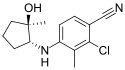
|
| MK-0773 (PF-05314882) | Steroid | GTx, Merck[30] | Sarcopenia, osteoporosis[18][30] | Phase II (discontinued)[18][30][31] | 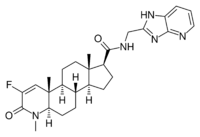
|
| MK-3984 | Benzylpropionamide | Merck | Sarcopenia[18] | Phase I[18] | 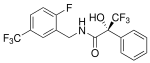
|
| OPK-88004 (LY-2452473, TT-701) | Indole | Eli Lilly, OPKO[32] | Benign prostatic hyperplasia, quality of life in prostate cancer, erectile dysfunction[32][33] | Phase II[32] | 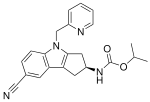
|
| PF-06260414 | Isoquinoline | Pfizer[34] | Cachexia[34] | Phase I (discontinued)[34] | 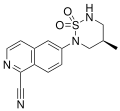
|
| PS-178990 | Unknown[18] | Bristol-Myers Squibb, Ligand Pharmaceuticals[35] | Andropause, cachexia[35][18] | Phase I (discontinued)[35][36][18] | Structure undisclosed[18] |
| Vosilasarm (RAD140, EP0062, testolone) | Phenyloxadiazole | Ellipsis[37] | Breast cancer, osteoporosis, sarcopenia[38] | Phase I/II[38] | Error creating thumbnail: Unable to save thumbnail to destination |
| YK-11 | Steroid | Toho University | Muscle wasting[39] | Preclinical | Error creating thumbnail: Unable to save thumbnail to destination |
Certain anabolic steroids, like trestolone, dimethandrolone undecanoate, and 11β-methyl-19-nortestosterone dodecylcarbonate, have also sometimes been classed as SARMs.[18]
Research and possible therapeutic applications
Due to their tissue selectivity, SARMs have the potential to treat a wide variety of conditions, including debilitating diseases. They have been investigated in human studies for the treatment of osteoporosis, cachexia, benign prostatic hyperplasia, stress urinary incontinence, prostate cancer, and breast cancer and have also been considered for the treatment of Alzheimer’s disease, Duchenne muscular dystrophy, hypogonadism and as a male contraceptive.[17][5] As of 2020[update], there are no SARMs which have been approved for therapeutic use by the United States Food and Drug Administration.[17]
Most SARMs have been tested in vitro or on rodents, while limited clinical trials in humans have been carried out.[4][40] Initial research focused on muscle wasting.[13] Enobosarm (ostarine) is the most well-studied SARM; according to its manufacturer, GTx Incorporated, 25 studies have been carried out on more than 1,700 humans as of 2020[update] involving doses from 1 to 18 mg each day.[41][12] As of 2020[update], there is little research distinguishing different SARMs from each other.[4] Much of the research on SARMs has been conducted by corporations and has not been made publicly available.[6]
Hypogonadism and hormone replacement therapy
Because of the potentially better side effect profile of SARMs compared to testosterone, SARMs have been proposed for use in the treatment of hypogonadism and for androgen replacement therapy.[42][43][17] Phase I and II trials have provided preliminary evidence that the SARMs enobosarm and GSK-2881078 (in elderly men and postmenopausal women), and OPL-88004 (prostate cancer survivors with low levels of testosterone) increase lean body mass and muscle size with little effect on the prostate, supporting the potential of SARMs for use in hormone replacement therapy.[9] However, it has been argued that SARMs are not ideal for use in androgen replacement therapy and could not replace testosterone in this context as they do not reproduce testosterone's full spectrum of effects, including androgenic potentiation via 5α-reduction and aromatization into estrogen.[44][45] Estrogenic signaling in particular is essential for normal male physiology and health, including for instance maintenance of bone strength.[46][47]
Benign prostatic hyperplasia
In rat models of benign prostatic hyperplasia (BPH), a condition where the prostate is enlarged in the absence of prostate cancer, SARMs reduced the weight of the prostate.[40] OPK-88004 advanced to a phase II trial in humans, but it was terminated due to difficulty in measuring prostate size, the trial's primary endpoint.[17]
Cancer
SARMs may help treat AR and estrogen receptor (ER) positive breast cancer, which comprise the majority of breast cancers.[5][48] AAS were historically used successfully to treat AR positive breast cancer, but were phased out after the development of antiestrogen therapies, due to androgenic side effects and concerns about aromatization to estrogen (which does not occur with SARMs).[48][13] Although a trial on AR positive triple negative breast cancer (which is ER-) was ended early due to lack of efficacy, enobosarm showed benefits in some patients with ER+, AR+ breast cancer in a phase II study. In patients with more than 40 percent AR positivity as determined by immunohistochemistry, the clinical benefit rate (CBR) was 80 percent and the objective response rate (ORR) was 48 percent—which was considered promising given that the patients had advanced disease and had been heavily pretreated.[49][48] In 2022, the FDA granted fast track designation to enobosarm for AR+, ER+, HER2- metastatic breast cancer.[50] Other SARMs such as vosilasarm have reached clinical trials in breast cancer patients.[37]
Bone and muscle wasting
As of 2020[update], there are no drugs approved to treat muscle wasting in people with chronic diseases, and there is therefore an unmet need for anabolic drugs with few side effects. One aspect hindering drug approval for treatments for cachexia and sarcopenia (two types of muscle wasting) is disagreement in what outcomes would demonstrate the efficacy of a drug. Several clinical trials have found that SARMs improve lean mass in humans, but it is not clear whether strength and physical function are also improved. After promising results in a phase II trial, a phase III trial of enobosarm was proven to increase lean body mass but did not show significant improvement in function. It and other drugs have been refused regulatory approval due to a lack of evidence that they increased physical performance; preventing decline in functionality was not considered an acceptable endpoint by the Food and Drug Administration. It is not known how SARMs interact with dietary protein intake and resistance training in people with muscle wasting.[12][17]
Phase II trials of enobosarm for stress urinary incontinence—considered promising, given that the levator ani muscle in the pelvic floor has a high androgen receptor density—did not meet their endpoint and were abandoned.[17][13]
Unlike other treatments for osteoporosis, which work by decreasing bone loss, SARMs have shown potential to promote growth in bone tissue. LY305 showed promising results in a phase I trial in humans.[17]
Side effects
In contrast to AAS and testosterone replacement, which have many side effects that have curtailed their medical use, SARMs are well tolerated and have mild and infrequent adverse events in randomized controlled trials.[40] SARMs are sometimes claimed to be non-virilizing (non-masculinizing).[17][51] In actuality however, SARMs are largely uncharacterized clinically in terms of potential virilizing effects.[4] In any case, as SARMs are not substrates for 5α-reductase and are not potentiated in 5α-reductase-expressing tissues like skin, hair follicles, and the prostate gland, they may be expected on a theoretical level to have reduced androgenic strength relative to testosterone in these tissues.[52][53] SARMs cannot be aromatized to estrogen, thus causing no estrogenic side effects, for instance gynecomastia.[54][17][5] Unlike most current forms of testosterone replacement, SARMs can be administered orally.[5]
SARM use can cause elevated liver enzymes and reduction in HDL cholesterol.[54][17] Transdermal administration via a skin patch may reduce these effects.[17][29] Several case reports have associated SARMs with hepatocellular drug-induced liver injury when used recreationally,[55] it is not known if the risk is significant for medical use.[40][5] Whether SARMs increase the risk of cardiovascular events is unknown.[40][5] SARMs have less effect on blood lipid profiles than testosterone replacement; it is not known whether androgen-induced HDL reductions increase cardiovascular risk; and SARMs increase insulin sensitivity and lower triglycerides.[5][12]
Although they cause less suppression of the hypothalamic–pituitary–gonadal axis (HPG axis) than testosterone, studies have found that gonadotropins, free and total testosterone, and SHBG can be reduced in a compound- and dose-dependent fashion in men from SARM usage.[4][12] Typically SHBG is reduced along with total testosterone and total cholesterol while hematocrit is increased. Most studies have found that follicle-stimulating hormone (FSH), luteinizing hormone (LH), prostate-specific antigen, estradiol, and DHT levels are not altered.[40] Of SARMs that have been investigated, enobosarm is one of the least suppressive of gonadotropins, even in doses much higher than used in clinical trials. How the HPG axis is affected in women using SARMs is unknown.[4][12] SARMs' effect in suppressing the gonadotropins FSH and LH is what makes SARMs potentially useful as a male contraceptive.[56]
Non-medical use
Outside of pharmaceutical research, SARMs are a gray market substance produced by small laboratories and often marketed as a research chemical supposedly not for human consumption.[4][57][58] Marketing SARMs for human consumption is illegal in some jurisdictions and has led to criminal convictions in the United States[59] and the largest-ever fine levied under Australia's Therapeutic Goods Act 1989.[60] Although SARMs are readily available for purchase on the internet, one study found that a majority of products advertised as SARMs online were mislabeled. Anecdotes and guides on usage can also be found online and on social media.[61][54][5] Some compounds are commonly marketed for recreational use as SARMs despite having a different mechanism of action. These substances include ibutamoren (MK-677), which increases secretion of growth hormone; GW501516 (cardarine), an exercise mimetic that works as an agonist of the PPARβ/δ; and SR9009 (Stenabolic), an agonist of the Rev-Erb, which plays a role in circadian rhythm.[4][62]
SARMs are used by bodybuilders and competitive athletes due to their anabolic and lack of androgenic effects,[5] particularly in the United States, Europe, and other western countries.[54] Some individuals using SARMs recreationally combine multiple SARMs or take a SARM along with other compounds, although there is no research on combining SARMs. The doses used often exceed those from clinical trials; nevertheless, the fat-free mass gained from SARMs is generally lower than what is obtained with moderate doses of testosterone derivatives.[4] According to one study of SARM users, more than 90 percent were satisfied with their usage and 64 percent would take SARMs again even though a majority experienced adverse effects.[63]
SARMs were banned by the World Anti-Doping Agency (WADA) in 2008.[4] SARMs can be detected in urine and hair after consumption.[64] WADA reported its first adverse analytical finding for SARMs in 2010 and the number of positive tests has increased since then; the most commonly detected SARMs are enobosarm (ostarine) and LGD-4033 (ligandrol).[65][66] Athletes competing in the NFL, NBA, UFC, NCAA, and the Olympics have tested positive.[55] There is limited evidence on how SARMs affect athletic performance.[67]
Terminology
SARMs are sometimes also referred to as "nonsteroidal androgens",[1][68] although not all SARMs are nonsteroidal in structure and steroidal SARMs also exist.[18] The first SARMs, discovered in 1998, which were nonsteroidal, were initially referred to as nonsteroidal androgens.[69] By 1999 however, on the basis of the selective estrogen receptor modulator (SERM)-like mixed agonist–antagonist and tissue-selective activity of these nonsteroidal androgen receptor agonists, the term "selective androgen receptor modulator" or "SARM" was introduced and started to be adopted.[42] Despite its widespread use, the term "selective androgen receptor modulator" has been criticized by some authors, like David Handelsman, who argue that it is a misleading pharmaceutical marketing term rather than an accurate pharmacological description.[44] He has also critiqued notions that SARMs isolate anabolic effects from so-called androgenic or virilizing effects, as has been previously claimed in the case of anabolic steroids.[44][70][71][72]
References
- ↑ 1.0 1.1 "Medicinal Use of Testosterone and Related Steroids Revisited". Molecules 26 (4): 1032. February 2021. doi:10.3390/molecules26041032. PMID 33672087. "SARMs are a novel group of compounds developed to selectively augment anabolic effects in muscles and bones, while avoiding undesirable androgenic effects in skin, larynx, and reproductive organs. The majority of these compounds lack the structural functionalities of the original anabolic steroids and are sometimes termed nonsteroidal androgens. It was hoped that these agents could be used in cases where conventional anabolic steroids produced undesirable side-effects, such as virilization in women and prostate hyperplasia in men [67]. Despite the enormous effort that has been expended in the development of selective anabolic agents, the androgenic effect is very hard to remove completely and many of the currently developed SARMs still do have some androgenic activity.".
- ↑ "An Overview of Next-Generation Androgen Receptor-Targeted Therapeutics in Development for the Treatment of Prostate Cancer". International Journal of Molecular Sciences 22 (4): 2124. February 2021. doi:10.3390/ijms22042124. PMID 33672769.
- ↑ "Structural Insights into Estrogen Receptors and Antiestrogen Therapies". Estrogen Receptor and Breast Cancer: Celebrating the 60th Anniversary of the Discovery of ER. Springer International Publishing. October 2018. ISBN 978-3-319-99350-8.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 Machek, Steven B.; Cardaci, Thomas D.; Wilburn, Dylan T.; Willoughby, Darryn S. (2020). "Considerations, possible contraindications, and potential mechanisms for deleterious effect in recreational and athletic use of selective androgen receptor modulators (SARMs) in lieu of anabolic androgenic steroids: A narrative review". Steroids 164: 108753. doi:10.1016/j.steroids.2020.108753. ISSN 0039-128X. PMID 33148520. https://www.sciencedirect.com/science/article/abs/pii/S0039128X20301793. "Sex-specific SARM effects on humans also remain considerably nebulous. SARMs may represent a more tempting option for female recreational use given potential previous tendencies towards less androgenic AAS (i.e. oxandrolone) [103]. Regardless, as the latter still imposes risk for permanent masculinization and hepatotoxicity, SARMs are largely uncharacterized for female-specific impacts.".
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 Solomon, Zachary J.; Mirabal, Jorge Rivera; Mazur, Daniel J.; Kohn, Taylor P.; Lipshultz, Larry I.; Pastuszak, Alexander W. (2019). "Selective Androgen Receptor Modulators: Current Knowledge and Clinical Applications". Sexual Medicine Reviews 7 (1): 84–94. doi:10.1016/j.sxmr.2018.09.006. PMID 30503797.
- ↑ 6.0 6.1 6.2 Jasuja, Ravi; Zacharov, Mikhail N.; Bhasin, Shalender (2012). "The state-of-the-art in the development of selective androgen receptor modulators". Testosterone: Action, Deficiency, Substitution (4 ed.). Cambridge University Press. pp. 459–460. ISBN 978-1-107-01290-5.
- ↑ 7.0 7.1 7.2 7.3 Bhasin, Shalender; Jasuja, Ravi (2009). "Selective Androgen Receptor Modulators (SARMs) as Function Promoting Therapies". Current Opinion in Clinical Nutrition and Metabolic Care 12 (3): 232–240. doi:10.1097/MCO.0b013e32832a3d79. ISSN 1363-1950. PMID 19357508.
- ↑ "Synthetic Anabolic Agents: Steroids and Nonsteroidal Selective Androgen Receptor Modulators". Doping in Sports. Handbook of Experimental Pharmacology. 195. 2010. pp. 99–126. doi:10.1007/978-3-540-79088-4_5. ISBN 978-3-540-79087-7. "One of the first synthetic analogs to testosterone prepared by the Noble laureate Ruzicka was 17α-methyltestosterone (Ruzicka et al. 1935)."
- ↑ 9.0 9.1 9.2 9.3 Bhasin, Shalender; Krishnan, Venkatesh; Storer, Thomas W; Steiner, Mitchell; Dobs, Adrian S (2023). "Androgens and Selective Androgen Receptor Modulators to Treat Functional Limitations Associated With Aging and Chronic Disease". The Journals of Gerontology: Series A 78 (Supplement_1): 25–31. doi:10.1093/gerona/glad027. PMID 37325955. PMC 10272983. https://academic.oup.com/biomedgerontology/article/78/Supplement_1/25/7199272#408664293.
- ↑ Katzung, Bertram G. (2017) (in en). Basic and Clinical Pharmacology 14th Edition. McGraw Hill Professional. p. 741. ISBN 978-1-259-64116-9.
- ↑ 11.0 11.1 11.2 11.3 Temerdashev, A. Z.; Dmitrieva, E. V. (2020). "Methods for the Determination of Selective Androgen Receptor Modulators". Journal of Analytical Chemistry 75 (7): 835–850. doi:10.1134/S1061934820070187.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 Fonseca, Guilherme Wesley Peixoto Da; Dworatzek, Elke; Ebner, Nicole; Von Haehling, Stephan (2020). "Selective androgen receptor modulators (SARMs) as pharmacological treatment for muscle wasting in ongoing clinical trials". Expert Opinion on Investigational Drugs 29 (8): 881–891. doi:10.1080/13543784.2020.1777275. PMID 32476495.
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 13.6 13.7 "Development of selective androgen receptor modulators (SARMs)". Molecular and Cellular Endocrinology 465: 134–142. April 2018. doi:10.1016/j.mce.2017.06.013. PMID 28624515.
- ↑ "Pharmacology of anabolic steroids". British Journal of Pharmacology 154 (3): 502–521. June 2008. doi:10.1038/bjp.2008.165. PMID 18500378.
- ↑ 15.0 15.1 "Coregulator function: a key to understanding tissue specificity of selective receptor modulators". Endocrine Reviews 25 (1): 45–71. February 2004. doi:10.1210/er.2003-0023. PMID 14769827.
- ↑ 16.0 16.1 16.2 "Androgen receptor: structure, role in prostate cancer and drug discovery". Acta Pharmacologica Sinica 36 (1): 3–23. January 2015. doi:10.1038/aps.2014.18. PMID 24909511.
- ↑ 17.00 17.01 17.02 17.03 17.04 17.05 17.06 17.07 17.08 17.09 17.10 17.11 Christiansen, Andrew R.; Lipshultz, Larry I.; Hotaling, James M.; Pastuszak, Alexander W. (March 2020). "Selective androgen receptor modulators: the future of androgen therapy?". Translational Andrology and Urology 9 (Suppl 2): S135–S148. doi:10.21037/tau.2019.11.02. ISSN 2223-4683. PMID 32257854.
- ↑ 18.00 18.01 18.02 18.03 18.04 18.05 18.06 18.07 18.08 18.09 18.10 18.11 18.12 18.13 18.14 18.15 "Overview of the development of selective androgen receptor modulators (SARMs) as pharmacological treatment for osteoporosis (1998-2021)". Eur J Med Chem 230: 114119. February 2022. doi:10.1016/j.ejmech.2022.114119. PMID 35063736.
- ↑ 19.0 19.1 19.2 "Andarine". AdisInsight. Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800019485.
- ↑ 20.0 20.1 20.2 "Arcarine". Synapse. PatSnap. https://synapse.patsnap.com/drug/3c0a1cb1cbc14552b622c39a85ef56fd.
- ↑ 21.0 21.1 21.2 "Enobosarm - Veru Healthcare". AdisInsight. Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800022562.
- ↑ 22.0 22.1 22.2 "DT 200". AdisInsight. Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800018432.
- ↑ 23.0 23.1 23.2 23.3 23.4 "GSK 971086". AdisInsight. Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800027354.
- ↑ 24.0 24.1 24.2 24.3 24.4 "GSK 2849466". AdisInsight. Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800036899.
- ↑ 25.0 25.1 25.2 "GSK 2881078". AdisInsight. Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800039743.
- ↑ Mohan, Divya; Rossiter, Harry; Watz, Henrik; Fogarty, Charles; Evans, Rachael A.; Man, William; Tabberer, Maggie; Beerahee, Misba et al. (1 March 2023). "Selective androgen receptor modulation for muscle weakness in chronic obstructive pulmonary disease: a randomised control trial" (in en). Thorax 78 (3): 258–266. doi:10.1136/thorax-2021-218360. ISSN 0040-6376. PMID 36283827. PMC 9985744. https://thorax.bmj.com/content/78/3/258.
- ↑ 27.0 27.1 27.2 "LGD 2941". AdisInsight. Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800013541.
- ↑ 28.0 28.1 28.2 "VK 5211". AdisInsight. Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800030373.
- ↑ 29.0 29.1 29.2 29.3 Krishnan, V.; Patel, N. J.; Mackrell, J. G.; Sweetana, S. A.; Bullock, H.; Ma, Y. L.; Waterhouse, T. H.; Yaden, B. C. et al. (2018). "Development of a selective androgen receptor modulator for transdermal use in hypogonadal patients". Andrology 6 (3): 455–464. doi:10.1111/andr.12479. PMID 29527831.
- ↑ 30.0 30.1 30.2 "MK 0773". AdisInsight. Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800024550.
- ↑ "A phase IIA randomized, placebo-controlled clinical trial to study the efficacy and safety of the selective androgen receptor modulator (SARM), MK-0773 in female participants with sarcopenia". J Nutr Health Aging 17 (6): 533–43. 2013. doi:10.1007/s12603-013-0335-x. PMID 23732550.
- ↑ 32.0 32.1 32.2 "OPK 88004". AdisInsight. Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800033580.
- ↑ Pencina, Karol M; Burnett, Arthur L; Storer, Thomas W; Guo, Wen; Li, Zhuoying; Kibel, Adam S; Huang, Grace; Blouin, Michelle et al. (2021). "A Selective Androgen Receptor Modulator (OPK-88004) in Prostate Cancer Survivors: A Randomized Trial". The Journal of Clinical Endocrinology & Metabolism 106 (8): 2171–2186. doi:10.1210/clinem/dgab361. PMID 34019661.
- ↑ 34.0 34.1 34.2 "PF 626414". AdisInsight. Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800040072.
- ↑ 35.0 35.1 35.2 "PS 178990". AdisInsight. Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800027228.
- ↑ "PS-178990". Synapse. PatSnap. https://synapse.patsnap.com/drug/78c027bec8a44220832e3922dbd38dfb.
- ↑ 37.0 37.1 Lim, Elgene; Hamilton, Erika; Palmieri, Carlo; Arkenau, Hendrik-Tobias; Brook, Sue; Fisher, Geoff; Mazur, Andrew (1 March 2023). "Abstract OT1-02-02: A phase 1/2 study to evaluate the safety and efficacy of EP0062, an oral Selective Androgen Receptor Modulator (SARM), for the treatment of AR+/HER2-/ER+ advanced breast cancer". Cancer Research 83 (5_Supplement): OT1–02–02-OT1-02-02. doi:10.1158/1538-7445.SABCS22-OT1-02-02.
- ↑ 38.0 38.1 "Vosilasarm - Ellipses Pharma". AdisInsight. Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800028398.
- ↑ Lee, Su Jin; Gharbi, Amal; Shin, Joo Eun; Jung, In Duk; Park, Yeong Min (2021). "Myostatin inhibitor YK11 as a preventative health supplement for bacterial sepsis". Biochemical and Biophysical Research Communications 543: 1–7. doi:10.1016/j.bbrc.2021.01.030. ISSN 0006-291X. PMID 33588136.
- ↑ 40.0 40.1 40.2 40.3 40.4 40.5 Sigalos, John T.; Walker, Dyvon T.; Lipschultz, Larry I. (2023). "Selective Androgen Receptor Modulators in the Treatment of Hypogonadism and Men's Health" (in en). Men's Reproductive and Sexual Health Throughout the Lifespan: An Integrated Approach to Fertility, Sexual Function, and Vitality. Cambridge University Press. p. 266. doi:10.1017/9781009197533.034. ISBN 978-1-009-19755-7.
- ↑ Zajac, Jeffrey D.; Seeman, Ego; Russell, Nicholas; Ramchand, Sabashini K.; Bretherton, Ingrid; Grossmann, Mathis; Davey, Rachel A. (2020). "Testosterone". Encyclopedia of Bone Biology. Academic Press. p. 545. ISBN 978-0-12-814082-6.
- ↑ 42.0 42.1 "Selective androgen receptor modulators (SARMs): a novel approach to androgen therapy for the new millennium". The Journal of Clinical Endocrinology and Metabolism 84 (10): 3459–3462. October 1999. doi:10.1210/jcem.84.10.6122. PMID 10522980. "We have chosen the term selective androgen receptor modulators (SARMs) after the terminology currently used for similar molecules targeting the estrogen receptor. ... Desired profile of activity of new SARMs: male applications: Selected indications may include glucocorticoid-induced osteoporosis, androgen replacement in elderly men, HIV-wasting, cancer cachexia, certain anemias, muscular dystrophies, and male contraception.".
- ↑ "Nuclear hormone receptors". Comprehensive Medicinal Chemistry II. Elsevier. 2007. pp. 993–1036. doi:10.1016/B0-08-045044-X/00063-8. ISBN 9780080450445. "A SARM for the treatment of hypogonadism or osteoporosis would be an AR agonist in the muscle and bone, with minimal hypertrophic agonist effects in the prostate."
- ↑ 44.0 44.1 44.2 "History of androgens and androgen action". Best Practice & Research. Clinical Endocrinology & Metabolism 36 (4): 101629. July 2022. doi:10.1016/j.beem.2022.101629. PMID 35277356. "The next invention was that of the first non-steroidal androgen by Dalton et al. [111] in 1998, six decades after the first non-steroidal estrogen [112]. This creates a new class of non-steroidal synthetic androgen, often termed Specific Androgen Receptor Modulators (SARM), a misleading marketing term rather than an accurate pharmacological description [113,114], usurping a speculative but unsound analogy with Specific Estrogen Receptor Modulators (SERM). [...] none of the non-steroidal androgens under development [116,117] are marketed by 2021. Yet hope springs eternal for this new attempt to separate anabolic from androgenic properties of androgens to facilitate marketing for muscle wasting and other selective effects of testosterone.".
- ↑ Feingold, K. R. et al. (5 October 2020). "Androgen Physiology, Pharmacology, Use and Misuse". Endotext. PMID 25905231. "These features suggest that non-steroidal androgens have potential for development into pharmacologic androgen therapy regimens as tissue-selective mixed or partial androgen agonists (“selective androgen receptor modulators”, SARM) (419, 718). Conversely, they are not ideal for androgen replacement therapy where the full spectrum of testosterone effects including aromatization is idealy required, especially for tissues such as the brain (148, 159) and bone (153) where aromatization is a prominent feature of testosterone action.".
- ↑ "MECHANISMS IN ENDOCRINOLOGY: Estradiol as a male hormone". Eur J Endocrinol 181 (1): R23–R43. July 2019. doi:10.1530/EJE-18-1000. PMID 31096185.
- ↑ "Estrogens in Male Physiology". Physiol Rev 97 (3): 995–1043. July 2017. doi:10.1152/physrev.00018.2016. PMID 28539434.
- ↑ 48.0 48.1 48.2 Dai, Charles; Ellisen, Leif W (2023). "Revisiting Androgen Receptor Signaling in Breast Cancer". The Oncologist 28 (5): 383–391. doi:10.1093/oncolo/oyad049. PMID 36972361. PMC 10166165. https://academic.oup.com/oncolo/article/28/5/383/7087214.
- ↑ Palmieri, Carlo; Linden, Hannah M.; Birrell, Stephen; Lim, Elgene; Schwartzberg, Lee S.; Rugo, Hope S.; Cobb, Patrick Wayne; Jain, Kirti et al. (2021). "Efficacy of enobosarm, a selective androgen receptor (AR) targeting agent, correlates with the degree of AR positivity in advanced AR+/estrogen receptor (ER)+ breast cancer in an international phase 2 clinical study." (in en). Journal of Clinical Oncology 39 (15_suppl): 1020. doi:10.1200/JCO.2021.39.15_suppl.1020. ISSN 0732-183X. https://ascopubs.org/doi/abs/10.1200/JCO.2021.39.15_suppl.1020.
- ↑ "FDA Grants Fast Track Designation to Enobosarm in AR+, ER+, HER2- Metastatic Breast Cancer" (in en). 10 January 2022. https://www.cancernetwork.com/view/fda-grants-fast-track-designation-to-enobosarm-in-ar-er-her2--metastatic-breast-cancer.
- ↑ "Deciphering the selective androgen receptor modulators paradigm". Expert Opin Drug Discov 8 (2): 191–218. February 2013. doi:10.1517/17460441.2013.741582. PMID 23231475.
- ↑ "Ockham's razor and selective androgen receptor modulators (SARMs): are we overlooking the role of 5α-reductase?". Mol Interv 7 (1): 10–3. February 2007. doi:10.1124/mi.7.1.3. PMID 17339601.
- ↑ "Intracrine and myotrophic roles of 5α-reductase and androgens: a review". Med Sci Sports Exerc 44 (5): 818–26. May 2012. doi:10.1249/MSS.0b013e31823bfcbf. PMID 21988936.
- ↑ 54.0 54.1 54.2 54.3 Xie, Youquan; Tian, Yucheng; Zhang, Yuming; Zhang, Zhisheng; Chen, Rui; Li, Mian; Tang, Jiawei; Bian, Jinlei et al. (15 February 2022). "Overview of the development of selective androgen receptor modulators (SARMs) as pharmacological treatment for osteoporosis (1998–2021)". European Journal of Medicinal Chemistry 230: 114119. doi:10.1016/j.ejmech.2022.114119. ISSN 0223-5234. PMID 35063736.
- ↑ 55.0 55.1 Hahamyan, Henrik; Gould, Heath; Gregory, Andrew; Dodson, Christopher; Gausden, Elizabeth; Voos, James; Calcei, Jacob; Vasireddi, Nikhil (2023). "Poster 390: Systematic Review of SARMs Abuse in Athletes" (in en). Orthopaedic Journal of Sports Medicine 11 (7_suppl3): 2325967123S00352. doi:10.1177/2325967123S00352. ISSN 2325-9671.
- ↑ Bhasin, Shalender (2015). "Selective Androgen Receptor Modulators as Function Promoting Therapies". The Journal of Frailty & Aging 4 (3): 121–122. doi:10.14283/jfa.2015.65. ISSN 2260-1341. PMID 27030938.
- ↑ Sobolevsky, Tim; Ahrens, Brian (2021). "High-throughput liquid chromatography tandem mass spectrometry assay as initial testing procedure for analysis of total urinary fraction" (in en). Drug Testing and Analysis 13 (2): 283–298. doi:10.1002/dta.2917. ISSN 1942-7603. PMID 32852861. https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/abs/10.1002/dta.2917.
- ↑ Turnock, Dr Luke; Gibbs, Dr Nick (2023). "Click, click, buy: The market for novel synthetic peptide hormones on mainstream e-commerce platforms in the UK". Performance Enhancement & Health 11 (2): 100251. doi:10.1016/j.peh.2023.100251. ISSN 2211-2669.
- ↑ Oberheiden, Nick (26 June 2023). "The FDA Continues to Crack Down on SARM Manufacturing and Distribution". https://federal-lawyer.com/the-fda-continues-to-crack-down-on-sarm-manufacturing-and-distribution.
- ↑ Jacobson, Hogan Lovells-Mandi; Zhang, Angell; Forrai, Zachary (3 August 2021). "Failure to remove unlawful advertising attracts $12 million penalty" (in en). Lexology. https://www.lexology.com/library/detail.aspx?g=311cfe98-f39e-415b-8a56-0fba4f091c5f.
- ↑ Hahamyan, Henrik A.; Vasireddi, Nikhil; Voos, James E.; Calcei, Jacob G. (2023). "Social media's impact on widespread SARMs abuse" (in en). The Physician and Sportsmedicine 51 (4): 291–293. doi:10.1080/00913847.2022.2078679. ISSN 0091-3847. PMID 35574698.
- ↑ Handschin, Christoph (2016). "Caloric restriction and exercise "mimetics: Ready for prime time?". Pharmacological Research 103: 158–166. doi:10.1016/j.phrs.2015.11.009. PMID 26658171.
- ↑ Efimenko, Iakov V.; Valancy, David; Dubin, Justin M.; Ramasamy, Ranjith (2022). "Adverse effects and potential benefits among selective androgen receptor modulators users: a cross-sectional survey" (in en). International Journal of Impotence Research 34 (8): 757–761. doi:10.1038/s41443-021-00465-0. ISSN 1476-5489. PMID 34471228.
- ↑ Kintz, Pascal; Ameline, Alice; Gheddar, Laurie; Raul, Jean-Sébastien (2019). "LGD-4033, S-4 and MK-2866 – Testing for SARMs in hair: About 2 doping cases". Toxicologie Analytique et Clinique 31 (1): 56–63. doi:10.1016/j.toxac.2018.12.001. ISSN 2352-0078. https://www.sciencedirect.com/science/article/abs/pii/S2352007818303275.
- ↑ "Selektive Androgenrezeptor-Modulatoren (SARMs)" (in de). https://www.dshs-koeln.de/institut-fuer-biochemie/doping-substanzen/doping-lexikon/s/selektive-androgenrezeptor-modulatoren-sarms/.
- ↑ Kintz, Pascal (5 January 2022). "The forensic response after an adverse analytical finding (doping) involving a selective androgen receptor modulator (SARM) in human athlete". Journal of Pharmaceutical and Biomedical Analysis 207: 114433. doi:10.1016/j.jpba.2021.114433. ISSN 1873-264X. PMID 34715583.
- ↑ Warrier, Alec A.; Azua, Eric N.; Kasson, Luke B.; Allahabadi, Sachin; Khan, Zeeshan A.; Mameri, Enzo S.; Swindell, Hasani W.; Tokish, John M. et al. (2023). "Performance-Enhancing Drugs in Healthy Athletes: An Umbrella Review of Systematic Reviews and Meta-analyses" (in en). Sports Health: A Multidisciplinary Approach. doi:10.1177/19417381231197389. ISSN 1941-7381. PMID 37688400. https://journals.sagepub.com/doi/abs/10.1177/19417381231197389.
- ↑ "Nonsteroidal selective androgen receptors modulators (SARMs): designer androgens with flexible structures provide clinical promise". Endocrinology 145 (12): 5417–9. December 2004. doi:10.1210/en.2004-1207. PMID 15545403.
- ↑ "Discovery of nonsteroidal androgens". Biochem Biophys Res Commun 244 (1): 1–4. March 1998. doi:10.1006/bbrc.1998.8209. PMID 9514878.
- ↑ "Commentary: androgens and "anabolic steroids": the one-headed janus". Endocrinology 152 (5): 1752–4. May 2011. doi:10.1210/en.2010-1501. PMID 21511988. "Although development of the first nonsteroidal androgens (17, 18) as candidate selective AR modulators (19) raises hope of resurrecting this defunct term (20), prereceptor activation mechanisms cannot apply to nonsteroidal androgens, and the singular AR lacks a dual drive mechanism of the other paired sex steroid receptors. Consequently, it is not surprising that available knowledge (21) provides only slender hope that this failed, and probably false, dichotomy will now succeed through a renewed search guided by the same in vivo bioassay.".
- ↑ "Androgen Misuse and Abuse". Endocr Rev 42 (4): 457–501. July 2021. doi:10.1210/endrev/bnab001. PMID 33484556. "However, a third major quest, for the development of a nonvirilizing androgen (“anabolic steroid”) suitable for use in women and children, based on dissociating the virilizing from the anabolic effects of androgens failed comprehensively (36). This failure is now understood as being due to the discovery of a singular androgen receptor (AR) together with the misinterpretation of nonspecific whole animal androgen bioassays employed to distinguish between anabolic and virilizing effects (37). The term “androgen” is used herein for both endogenous and synthetic androgens including references to chemicals named elsewhere as “anabolic steroids,” “anabolic-androgenic steroids,” or “specific AR modulators” (SARM), which continue to make an obsolete and oxymoronic distinction between virilizing and anabolic effects of androgens where there is no difference (36).".
- ↑ Handelsman, David J. (2012-07-26). "Androgen therapy in non-gonadal disease". Testosterone. Cambridge University Press. pp. 372–407. doi:10.1017/cbo9781139003353.018. ISBN 978-1-139-00335-3. "The development of nonsteroidal androgens, marketed as “selective androgen receptor modulators” (SARMs), offers new possibilities for adjuvant pharmacological androgen therapy. In contrast to the full spectrum of androgen effects of testosterone, such SARMs would be pure androgens not subject to tissue-specific activation by aromatization to a corresponding estrogen or to amplification of androgenic potency by 5α-reduction. In this context the endogenous pure androgens nandrolone and DHT can be considered prototype SARMs. SARMs are not the modern embodiment of so-called “anabolic steroids,” an outdated term referring to hypothetical but nonexistent non-virilizing androgens targeted exclusively to muscle, a failed concept lacking biological proof of principle (Handelsman 2011)."
{{Navbox
| name = Androgens and antiandrogens | title = Androgens and antiandrogens | state = collapsed | listclass = hlist | groupstyle = text-align:center;
| group1 = Androgens
(incl. AAS)
| list1 =
| group2 = Antiandrogens | list2 = {{Navbox|child | groupstyle = text-align:center; | groupwidth = 9em;
| group1 = AR antagonists | list1 =
- Steroidal: Abiraterone acetate
- Canrenone
- Chlormadinone acetate
- Cyproterone acetate
- Delmadinone acetate
- Dienogest
- Drospirenone
- Medrogestone
- Megestrol acetate
- Nomegestrol acetate
- Osaterone acetate
- Oxendolone
- Potassium canrenoate
- Spironolactone
- Nonsteroidal: Apalutamide
- Bicalutamide
- Cimetidine
- Darolutamide
- Enzalutamide
- Flutamide
- Ketoconazole
- Nilutamide
- Seviteronel†
- Topilutamide (fluridil)
| group2 = Steroidogenesis| list2 =
inhibitors
| 5α-Reductase | |
|---|---|
| Others |
| group3 = Antigonadotropins | list3 =
- D2 receptor antagonists (prolactin releasers) (e.g., domperidone, metoclopramide, risperidone, haloperidol, chlorpromazine, sulpiride)
- Estrogens (e.g., bifluranol, [[diethylstilbestrol, estradiol, estradiol esters, ethinylestradiol, ethinylestradiol sulfonate, paroxypropione)
- GnRH agonists (e.g., leuprorelin)
- GnRH antagonists (e.g., cetrorelix)
- Progestogens (incl., chlormadinone acetate, [[cyproterone acetate, hydroxyprogesterone caproate, gestonorone caproate, [[Chemistry:Medroxyprogesterone medroxyprogesterone acetate, Chemistry:Megestrol acetate|megestrol acetate]])
| group4 = Others | list4 =
- Androstenedione immunogens: Androvax (androstenedione albumin)
- Ovandrotone albumin (Fecundin)
}}
| liststyle = background:#DDDDFF;| list3 =
- #WHO-EM
- ‡Withdrawn from market
- Clinical trials:
- †Phase III
- §Never to phase III
- See also
- Androgen receptor modulators
- Estrogens and antiestrogens
- Progestogens and antiprogestogens
- List of androgens/anabolic steroids
}}
 |
 KSF
KSF