Serotonin
Topic: Chemistry
 From HandWiki - Reading time: 58 min
From HandWiki - Reading time: 58 min
Serotonin (/ˌsɛrəˈtoʊnɪn, ˌsɪərə-/)[1][2][3], also known as 5-hydroxytryptamine (5-HT), is a monoamine neurotransmitter with a wide range of functions in both the central nervous system (CNS) and also peripheral tissues. It is involved in mood, cognition, reward, learning, memory, and physiological processes such as vomiting and vasoconstriction.[4] In the CNS, serotonin regulates mood, appetite, and sleep.[5][6][unreliable medical source]
Most of the body's serotonin—about 90%—is synthesized in the gastrointestinal tract by enterochromaffin cells, where it regulates intestinal movements.[7][8][9] It is also produced in smaller amounts in the brainstem's raphe nuclei, the skin's Merkel cells, pulmonary neuroendocrine cells, and taste receptor cells of the tongue. Once secreted, serotonin is taken up by platelets in the blood, which release it during clotting to promote vasoconstriction and platelet aggregation.[10] Around 8% of the body's serotonin is stored in platelets, and 1–2% is found in the CNS.[11]
Serotonin acts as both a vasoconstrictor and vasodilator depending on concentration and context, influencing hemostasis and blood pressure regulation.[12] It plays a role in stimulating myenteric neurons and enhancing gastrointestinal motility through uptake and release cycles in platelets and surrounding tissue.[13] Biochemically, serotonin is an indoleamine synthesized from tryptophan and metabolized primarily in the liver to 5-hydroxyindoleacetic acid (5-HIAA).
Serotonin is targeted by several classes of antidepressants, including selective serotonin reuptake inhibitors (SSRIs) and serotonin–norepinephrine reuptake inhibitors (SNRIs), which block reabsorption in the synapse to elevate its levels. It is found in nearly all bilateral animals, including insects, spiders and worms,[14] and also occurs in fungi and plants.[15] In plants and insect venom, it serves a defensive function by inducing pain.[16] Serotonin released by pathogenic amoebae may cause diarrhea in the human gut,[17] while its presence in seeds and fruits is thought to stimulate digestion and facilitate seed dispersal.[18]
Molecular structure
Biochemically, the indoleamine molecule derives from the amino acid tryptophan, via the (rate-limiting) hydroxylation of the 5 position on the ring (forming the intermediate 5-hydroxytryptophan), and then decarboxylation to produce serotonin.[19] Preferable conformations are defined via ethylamine chain, resulting in six different conformations.[20]
Crystal structure
Serotonin crystallizes in P212121 chiral space group forming different hydrogen-bonding interactions between serotonin molecules via N-H...O and O-H...N intermolecular bonds.[21] Serotonin also forms several salts, including pharmaceutical formulation of serotonin adipate.[22]
Biological role
Serotonin is involved in numerous physiological processes,[23] including sleep,[24] thermoregulation, learning and memory, pain, (social) behavior,[25] sexual activity, feeding, motor activity, neural development,[26] and biological rhythms.[27] In less complex animals, such as some invertebrates, serotonin regulates feeding and other processes.[28] In plants serotonin synthesis seems to be associated with stress signals.[15][29] Despite its longstanding prominence in pharmaceutical advertising, the claim that low serotonin levels cause depression is not supported by scientific evidence.[30][31][32]
Cellular effects
Serotonin primarily acts through its receptors and its effects depend on which cells and tissues express these receptors.[27]
Metabolism involves first oxidation by monoamine oxidase to 5-hydroxyindoleacetaldehyde (5-HIAL).[33][34] The rate-limiting step is hydride transfer from serotonin to the flavin cofactor.[35] There follows oxidation by aldehyde dehydrogenase (ALDH) to 5-hydroxyindoleacetic acid (5-HIAA), the indole acetic-acid derivative. The latter is then excreted by the kidneys.
Receptors
The serotonin receptors are located on the cell membrane of nerve cells and other cell types in animals, and mediate the effects of serotonin as the endogenous ligand and of a broad range of pharmaceutical and psychedelic drugs. There are currently 14 known serotonin receptors, including the serotonin 5-HT1 (1A, 1B, 1D, 1E, 1F), 5-HT2 (2A, 2B, 2C), 5-HT3, 5-HT4, 5-HT5 (5A, 5B), 5-HT6, and 5-HT7 receptors. Except for the serotonin 5-HT3 receptor, a ligand-gated ion channel, all other 5-HT receptors are G-protein-coupled receptors (also called seven-transmembrane, or heptahelical receptors) that activate an intracellular second messenger cascade.[36] The 5-HT5B receptor is present in rodents but not in humans.
In addition to the serotonin receptors, serotonin is an agonist of the trace amine-associated receptor 1 (TAAR1) in some species.[37][38] It is a weak TAAR1 partial agonist in rats, but is inactive at the TAAR1 in mice and humans.[37][38]
The cryo-EM structures of the serotonin 5-HT2A receptor with serotonin, as well as with various serotonergic psychedelics, have been solved and published by Bryan L. Roth and colleagues.[39][40]
Termination
Serotonergic action is terminated primarily via uptake of 5-HT from the synapse. This is accomplished through the specific monoamine transporter for 5-HT, SERT, on the presynaptic neuron. Various agents can inhibit 5-HT reuptake, including cocaine, dextromethorphan (an antitussive), tricyclic antidepressants and selective serotonin reuptake inhibitors (SSRIs). A 2006 study found that a significant portion of 5-HT's synaptic clearance is due to the selective activity of the plasma membrane monoamine transporter (PMAT) which actively transports the molecule across the membrane and back into the presynaptic cell.[41]
In contrast to the high affinity of SERT, the PMAT has been identified as a low-affinity transporter, with an apparent Km of 114 micromoles/l for serotonin, which is approximately 230 times higher than that of SERT. However, the PMAT, despite its relatively low serotonergic affinity, has a considerably higher transport "capacity" than SERT, "resulting in roughly comparable uptake efficiencies to SERT ... in heterologous expression systems."[41] The study also suggests that the administration of SSRIs such as fluoxetine and sertraline may be associated with an inhibitory effect on PMAT activity when used at higher than normal dosages (IC50 test values used in trials were 3–4 fold higher than typical prescriptive dosage).
Serotonylation
Serotonin can also signal through a nonreceptor mechanism called serotonylation, in which serotonin modifies proteins.[42] This process underlies serotonin's effects upon platelet-forming cells (thrombocytes) in which it links to the modification of signaling enzymes called GTPases that then trigger the release of vesicle contents by exocytosis.[43] A similar process underlies the pancreatic release of insulin.[42]
The effects of serotonin upon vascular smooth muscle tone – the biological function after which serotonin was originally named – depend upon the serotonylation of proteins involved in the contractile apparatus of muscle cells.[44]
| Receptor | Ki (nM)[45] | Receptor function[Note 1] |
|---|---|---|
| 5-HT1 receptor family signals via Gi/o inhibition of adenylyl cyclase. | ||
| 5-HT1A | 3.17 | date=March 2014} agonists ↓); depression (agonists ↓); positive, negative, and cognitive symptoms of schizophrenia (partial agonists ↓); analgesia (agonists ↑); aggression (agonists ↓); dopamine release in the prefrontal cortex (agonists ↑); serotonin release and synthesis (agonists ↓) |
| 5-HT1B | 4.32 | Vasoconstriction (agonists ↑); aggression (agonists ↓); bone mass (↓). Serotonin autoreceptor. |
| 5-HT1D | 5.03 | Vasoconstriction (agonists ↑) |
| 5-HT1E | 7.53 | |
| 5-HT1F | 10 | |
| 5-HT2 receptor family signals via Gq activation of phospholipase C. | ||
| 5-HT2A | 11.55 | Psychedelia (agonists ↑); depression (agonists & antagonists ↓); anxiety (antagonists ↓); positive and negative symptoms of schizophrenia (antagonists ↓); norepinephrine release from the locus coeruleus (antagonists ↑); glutamate release in the prefrontal cortex (agonists ↑); dopamine in the prefrontal cortex (agonists ↑);[46] urinary bladder contractions (agonists ↑)[47] |
| 5-HT2B | 8.71 | Cardiovascular functioning (agonists increase risk of pulmonary hypertension), empathy (via von Economo neurons[48]) |
| 5-HT2C | 5.02 | Dopamine release into the mesocorticolimbic pathway (agonists ↓); acetylcholine release in the prefrontal cortex (agonists ↑); dopaminergic and noradrenergic activity in the frontal cortex (antagonists ↑);[49] appetite (agonists ↓); antipsychotic effects (agonists ↑); antidepressant effects (agonists & antagonists ↑) |
| Other 5-HT receptors | ||
| 5-HT3 | 593 | Emesis (agonists ↑); anxiolysis (antagonists ↑). |
| 5-HT4 | 125.89 | Movement of food across the GI tract (agonists ↑); memory & learning (agonists ↑); antidepressant effects (agonists ↑). Signalling via Gαs activation of adenylyl cyclase. |
| 5-HT5A | 251.2 | Memory consolidation.[50] Signals via Gi/o inhibition of adenylyl cyclase. |
| 5-HT6 | 98.41 | Cognition (antagonists ↑); antidepressant effects (agonists & antagonists ↑); anxiogenic effects (antagonists ↑[51]). Gs signalling via activating adenylyl cyclase. |
| 5-HT7 | 8.11 | Cognition (antagonists ↑); antidepressant effects (antagonists ↑). Acts by Gs signalling via activating adenylyl cyclase. |
Nervous system
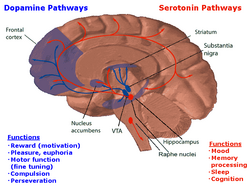
The neurons of the raphe nuclei are the principal source of 5-HT release in the brain.[52] There are nine raphe nuclei, designated B1–B9, which contain the majority of serotonin-containing neurons (some scientists chose to group the nuclei raphes lineares into one nucleus), all of which are located along the midline of the brainstem, and centered on the reticular formation.[53][54] Axons from the neurons of the raphe nuclei form a neurotransmitter system reaching almost every part of the central nervous system. Axons of neurons in the lower raphe nuclei terminate in the cerebellum and spinal cord, while the axons of the higher nuclei spread out in the entire brain.
It is the dorsal part of the raphe nucleus that contains neurons projecting to the central nervous system. Serotonin-releasing neurons in this area receive input from a large number of areas, notably from prefrontal cortex, lateral habenula, preoptic area, substantia nigra and amygdala.[55] These neurons are thought to communicate the expectation of rewards in the near future, a quantity called state value in reinforcement learning.[56]
Ultrastructure and function
The serotonin nuclei may also be divided into two main groups, the rostral and caudal containing three and four nuclei respectively. The rostral group consists of the caudal linear nuclei (B8), the dorsal raphe nuclei (B6 and B7) and the median raphe nuclei (B5, B8 and B9), that project into multiple cortical and subcortical structures. The caudal group consists of the nucleus raphe magnus (B3), raphe obscurus nucleus (B2), raphe pallidus nucleus (B1), and lateral medullary reticular formation, that project into the brainstem.[57]
The serotonergic pathway is involved in sensorimotor function, with pathways projecting both into cortical (Dorsal and Median Raphe Nuclei), subcortical, and spinal areas involved in motor activity. Pharmacological manipulation suggests that serotonergic activity increases with motor activity while firing rates of serotonergic neurons increase with intense visual stimuli. Animal models suggest that kainate signaling negatively regulates serotonin actions in the retina, with possible implications for the control of the visual system.[58] The descending projections form a pathway that inhibits pain called the "descending inhibitory pathway" that may be relevant to a disorder such as fibromyalgia, migraine, and other pain disorders, and the efficacy of antidepressants in them.[59]
Serotonergic projections from the caudal nuclei are involved in regulating mood and emotion, and hypo-[60] or hyper-serotonergic[61] states may be involved in depression and sickness behavior.
Microanatomy
Serotonin is released into the synapse, or space between neurons, and diffuses over a relatively wide gap (>20 nm) to activate 5-HT receptors located on the dendrites, cell bodies, and presynaptic terminals of adjacent neurons.
When humans smell food, dopamine is released to increase the appetite. But, unlike in worms, serotonin does not increase anticipatory behaviour in humans; instead, the serotonin released while consuming activates 5-HT2C receptors on dopamine-producing cells. This halts their dopamine release, and thereby serotonin decreases appetite. Drugs that block 5-HT2C receptors make the body unable to recognize when it is no longer hungry or otherwise in need of nutrients, and are associated with weight gain,[62] especially in people with a low number of receptors.[63] The expression of 5-HT2C receptors in the hippocampus follows a diurnal rhythm,[64] just as the serotonin release in the ventromedial nucleus, which is characterised by a peak at morning when the motivation to eat is strongest.[65]
In macaques, alpha males have twice the level of serotonin in the brain as subordinate males and females (measured by the concentration of 5-HIAA in the cerebrospinal fluid (CSF)). Dominance status and CSF serotonin levels appear to be positively correlated. When dominant males were removed from such groups, subordinate males begin competing for dominance. Once new dominance hierarchies were established, serotonin levels of the new dominant individuals also increased to double those in subordinate males and females. The reason why serotonin levels are only high in dominant males, but not dominant females has not yet been established.[66]
In humans, levels of 5-HT1A receptor inhibition in the brain show negative correlation with aggression,[67] and a mutation in the gene that codes for the 5-HT2A receptor may double the risk of suicide for those with that genotype.[68] Serotonin in the brain is not usually degraded after use, but is collected by serotonergic neurons by serotonin transporters on their cell surfaces. Studies have revealed nearly 10% of total variance in anxiety-related personality depends on variations in the description of where, when and how many serotonin transporters the neurons should deploy.[69]
Outside the nervous system
Digestive tract (emetic)
Serotonin regulates gastrointestinal (GI) function. The gut is surrounded by enterochromaffin cells, which release serotonin in response to food in the lumen. This makes the gut contract around the food. Platelets in the veins draining the gut collect excess serotonin. There are often serotonin abnormalities in gastrointestinal disorders such as constipation and irritable bowel syndrome.[70]
If irritants are present in the food, the enterochromaffin cells release more serotonin to make the gut move faster, i.e., to cause diarrhea, so the gut is emptied of the noxious substance. If serotonin is released in the blood faster than the platelets can absorb it, the level of free serotonin in the blood is increased. This activates 5-HT3 receptors in the chemoreceptor trigger zone that stimulate vomiting.[71] Thus, drugs and toxins stimulate serotonin release from enterochromaffin cells in the gut wall which can induce emesis. The enterochromaffin cells not only react to bad food but are also very sensitive to irradiation and cancer chemotherapy. Drugs that block 5HT3 are very effective in controlling the nausea and vomiting produced by cancer treatment, and are considered the gold standard for this purpose.[72]
Lungs
The lung,[73] including that of reptiles,[74] contains specialized epithelial cells that occur as solitary cells or as clusters called neuroepithelial bodies or bronchial Kulchitsky cells or alternatively K cells.[75] These are enterochromaffin cells that like those in the gut release serotonin.[75] Their function is probably vasoconstriction during hypoxia.[73]
Skin
Serotonin is also produced by Merkel cells which are part of the somatosensory system.[76]
Bone metabolism
In mice and humans, alterations in serotonin levels and signalling have been shown to regulate bone mass.[77][78][79][80] Mice that lack brain serotonin have osteopenia, while mice that lack gut serotonin have high bone density. In humans, increased blood serotonin levels have been shown to be a significant negative predictor of low bone density. Serotonin can also be synthesized, albeit at very low levels, in the bone cells. It mediates its actions on bone cells using three different receptors. Through 5-HT1B receptors, it negatively regulates bone mass, while it does so positively through 5-HT2B receptors and 5-HT2C receptors. There is very delicate balance between physiological role of gut serotonin and its pathology. Increase in the extracellular content of serotonin results in a complex relay of signals in the osteoblasts culminating in FoxO1/ Creb and ATF4 dependent transcriptional events.[81] Following the 2008 findings that gut serotonin regulates bone mass, the mechanistic investigations into what regulates serotonin synthesis from the gut in the regulation of bone mass have started. Piezo1 has been shown to sense RNA in the gut and relay this information through serotonin synthesis to the bone by acting as a sensor of single-stranded RNA (ssRNA) governing 5-HT production. Intestinal epithelium-specific deletion of mouse Piezo1 profoundly disturbed gut peristalsis, impeded experimental colitis, and suppressed serum 5-HT levels. Because of systemic 5-HT deficiency, conditional knockout of Piezo1 increased bone formation. Notably, fecal ssRNA was identified as a natural Piezo1 ligand, and ssRNA-stimulated 5-HT synthesis from the gut was evoked in a MyD88/TRIF-independent manner. Colonic infusion of RNase A suppressed gut motility and increased bone mass. These findings suggest gut ssRNA as a master determinant of systemic 5-HT levels, indicating the ssRNA-Piezo1 axis as a potential prophylactic target for treatment of bone and gut disorders. Studies in 2008, 2010 and 2019 have opened the potential for serotonin research to treat bone mass disorders.[82][83]
Organ development
Since serotonin signals resource availability it is not surprising that it affects organ development. Many human and animal studies have shown that nutrition in early life can influence, in adulthood, such things as body fatness, blood lipids, blood pressure, atherosclerosis, behavior, learning, and longevity.[84][85][86] Rodent experiment shows that neonatal exposure to SSRIs makes persistent changes in the serotonergic transmission of the brain resulting in behavioral changes,[87][88] which are reversed by treatment with antidepressants.[89] By treating normal and knockout mice lacking the serotonin transporter with fluoxetine scientists showed that normal emotional reactions in adulthood, like a short latency to escape foot shocks and inclination to explore new environments were dependent on active serotonin transporters during the neonatal period.[90][91]
Human serotonin can also act as a growth factor directly. Liver damage increases cellular expression of 5-HT2A and 5-HT2B receptors, mediating liver compensatory regrowth (see Liver § Regeneration and transplantation)[92] Serotonin present in the blood then stimulates cellular growth to repair liver damage.[93]
5-HT2B receptors also activate osteocytes, which build up bone[94] However, serotonin also inhibits osteoblasts, through 5-HT1B receptors.[95]
Cardiovascular growth factor
Serotonin, in addition, evokes endothelial nitric oxide synthase activation and stimulates, through a 5-HT1B receptor-mediated mechanism, the phosphorylation of p44/p42 mitogen-activated protein kinase activation in bovine aortic endothelial cell cultures.[clarification needed][96] In blood, serotonin is collected from plasma by platelets, which store it. It is thus active wherever platelets bind in damaged tissue, as a vasoconstrictor to stop bleeding, and also as a fibrocyte mitotic (growth factor), to aid healing.[97]
Adipose tissue
Serotonin also regulates white and brown adipose tissue function, and adipocytes are capable of producing 5-HT separately from the gut. Serotonin increases lipogenesis through HTR2A in white adipose tissue, and suppressed thermogenesis in brown adipose tissue via Htr3.[98]
Pharmacology
Several classes of drugs target the serotonin system, including some antidepressants, anxiolytics, antipsychotics, analgesics, antimigraine drugs, oxytocics, antiemetics, appetite suppressants, and anticonvulsants, as well as psychedelics and entactogens.
Mechanism of action
At rest, serotonin is stored within the vesicles of presynaptic neurons. When stimulated by nerve impulses, serotonin is released as a neurotransmitter into the synapse, reversibly binding to the postsynaptic receptor to induce a nerve impulse on the postsynaptic neuron. Serotonin can also bind to auto-receptors on the presynaptic neuron to regulate the synthesis and release of serotonin. Normally serotonin is taken back into the presynaptic neuron to stop its action, then reused or broken down by monoamine oxidase.[99]
Antidepressants
Drugs that alter serotonin levels are used in treating depression, generalized anxiety disorder, and social phobia. Monoamine oxidase inhibitors (MAOIs) prevent the breakdown of monoamine neurotransmitters (including serotonin), and therefore increase concentrations of the neurotransmitter in the brain. MAOI therapy is associated with many adverse drug reactions, and patients are at risk of hypertensive emergency triggered by foods with high tyramine content, and certain drugs. Some drugs inhibit the re-uptake of serotonin, making it stay in the synaptic cleft longer. The tricyclic antidepressants (TCAs) inhibit the reuptake of both serotonin and norepinephrine. The newer selective serotonin reuptake inhibitors (SSRIs) have fewer side-effects and fewer interactions with other drugs.[100]
Certain SSRI medications have been shown to lower serotonin levels below the baseline after chronic use, despite initial increases.[101] The 5-HTTLPR gene codes for the number of serotonin transporters in the brain, with more serotonin transporters causing decreased duration and magnitude of serotonergic signaling.[102] The 5-HTTLPR polymorphism (l/l) causing more serotonin transporters to be formed is also found to be more resilient against depression and anxiety.[103][104]
Besides their use in treating depression and anxiety, certain serotonergic antidepressants are also approved and used to treat fibromyalgia, neuropathic pain, and chronic fatigue syndrome.[105][106]
Anxiolytics
Azapirone anxiolytics like buspirone and tandospirone act as serotonin 5-HT1A receptor agonists.[107][108]
Antipsychotics
Many antipsychotics bind to and modulate serotonin receptors, including the serotonin 5-HT1A, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT6, and 5-HT7 receptors, among others.[109][110] Activation of serotonin 5-HT1A receptors and blockade of serotonin 5-HT2A receptors may contribute to the therapeutic antipsychotic effects of these agents, whereas antagonism of serotonin 5-HT2C receptors has been especially implicated in side effects of antipsychotics.[109][110]
Antimigraine agents
Antimigraine agents such as the triptans like sumatriptan act as agonists of the serotonin 5-HT1B, 5-HT1D, and/or 5-HT1F receptors.[111][112] Earlier antimigraine agents were the ergoline derivatives and ergot-related drugs such as ergotamine, dihydroergotamine, and methysergide, which act as non-selective serotonin receptor agonists.[112][113][114]
Oxytocics
Certain lysergamides like ergometrine and methylergometrine are used clinically as oxytocic agents.[115][116] The oxytocic effects of these drugs are thought to most likely be mediated by agonism of serotonin 5-HT2 receptors in uterine smooth muscle tissue.[115][116]
Antiemetics
Some serotonin 5-HT3 receptor antagonists, such as ondansetron, granisetron, and tropisetron, are important antiemetic agents.[117][118] They are particularly important in treating the nausea and vomiting that occur during anticancer chemotherapy using cytotoxic drugs.[118] Another application is in the treatment of postoperative nausea and vomiting.[117]
Appetite suppressants
Some serotonin releasing agents, serotonin reuptake inhibitors, and/or serotonin 5-HT2C receptor agonists, such as fenfluramine, dexfenfluramine, chlorphentermine, sibutramine, and lorcaserin, have been approved and used as appetite suppressants for purposes of weight loss in the treatment of overweightness or obesity.[119][120][121][122][123] Several of the preceding agents have been withdrawn from the market due to toxicity, such as cardiac fibrosis or pulmonary hypertension.[123]
Anticonvulsants
Although it was previously withdrawn from the market as an appetite suppressant, fenfluramine was reintroduced as an anticonvulsant for treatment of seizures in certain rare forms of epilepsy like Dravet syndrome and Lennox–Gastaut syndrome.[124] Selective serotonin 5-HT2C receptor agonists, like lorcaserin, bexicaserin, and BMB-101, are also being developed for this use.[124][125][126][127]
Psychedelics
Serotonergic psychedelics, including drugs like psilocybin (found in psilocybin mushrooms), dimethyltryptamine (DMT) (found in ayahuasca), lysergic acid diethylamide (LSD), mescaline (found in peyote cactus), and 5-MeO-DMT (found in Anadenanthera trees and the Bufo alvarius toad), are non-selective agonists of the serotonin receptors and mediate their hallucinogenic effects specifically by activation of the serotonin 5-HT2A receptor.[128][129][130] This is evidenced by the fact that serotonin 5-HT2A receptor antagonists and so-called "trip killers" like ketanserin block the hallucinogenic effects of serotonergic psychedelics in humans, among many other findings.[128][129][131] Some serotonergic psychedelics, like psilocin, DMT, and 5-MeO-DMT, are substituted tryptamines and are very similar in chemical structure to serotonin.[130]
Serotonin itself, despite acting as a serotonin 5-HT2A receptor agonist, is thought to be non-hallucinogenic.[132] The hallucinogenic effects of serotonergic psychedelics appear to be mediated by activation of serotonin 5-HT2A receptors expressed in a population of cortical neurons in the medial prefrontal cortex (mPFC).[133][132] These serotonin 5-HT2A receptors, unlike most serotonin and related receptors, are expressed intracellularly.[133][132] In addition, the neurons containing them lack expression of the serotonin transporter (SERT), which normally transports serotonin from the extracellular space to the intracellular space within neurons.[133][132] Serotonin itself is too hydrophilic to enter serotonergic neurons without the SERT, and hence these serotonin 5-HT2A receptors are inaccessible to serotonin.[133][132] Conversely, serotonergic psychedelics are more lipophilic than serotonin and readily enter these neurons.[133][132] In addition to explaining why serotonin does not show psychedelic effects, these findings may explain why drugs that increase serotonin levels, like selective serotonin reuptake inhibitors (SSRIs) and various other types of serotonergic agents, do not produce psychedelic effects.[133][132] Artificial expression of the SERT in these medial prefrontal cortex neurons resulted in the serotonin releasing agent para-chloroamphetamine (PCA), which does not normally show psychedelic-like effects, being able to produce psychedelic-like effects in animals.[132]
Although serotonin itself is non-hallucinogenic, administration of very high doses of a serotonin precursor, like tryptophan or 5-hydroxytryptophan (5-HTP), or intracerebroventricular injection of high doses of serotonin directly into the brain, can produce psychedelic-like effects in animals.[134][135][136] These psychedelic-like effects can be abolished by indolethylamine N-methyltransferase (INMT) inhibitors, which block conversion of serotonin and other endogenous tryptamines into N-methylated tryptamines, including N-methylserotonin (NMS; norbufotenin), bufotenin (5-hydroxy-N,N-dimethyltryptamine; 5-HO-DMT), N-methyltryptamine (NMT), and N,N-dimethyltryptamine (DMT).[135][137][136] These N-methyltryptamines are much more lipophilic than serotonin and, in contrast, are able to diffuse into serotonergic neurons and activate intracellular serotonin 5-HT2A receptors.[135][136][133][132] Another possible metabolite of serotonin with psychedelic-like effects in animals is 5-methoxytryptamine (5-MT).[138][139][140]
DMT is a naturally occurring endogenous compound in the body.[141][142][143] In relation to the fact that serotonin itself is unable to activate intracellular serotonin 5-HT2A receptors, it is possible that DMT might be the endogenous ligand of these receptors rather than serotonin.[133][132]
Entactogens
The entactogen MDMA is a serotonin releasing agent and, while it also possesses other actions such as concomitant release of norepinephrine and dopamine and weak direct agonism of the serotonin 5-HT2 receptors, its serotonin release plays a key role in its unique entactogenic effects.[144] Entactogens like MDMA should be distinguished from other drugs such as stimulants like amphetamine and psychedelics like LSD, although MDMA itself also has some characteristics of both of these types of agents.[144][145] Coadministration of selective serotonin reuptake inhibitors (SSRIs), which block the serotonin transporter (SERT) and prevent MDMA from inducing serotonin release, markedly reduce the subjective effects of MDMA, demonstrating the key role of serotonin in the effects of the drug.[146] Serotonin releasing agents like MDMA achieve much greater increases in serotonin levels than SSRIs and have far more robust of subjective effects.[147][148][149][150] Besides MDMA, many other entactogens also exist and are known.[151][152][145]
Serotonin syndrome
Extremely high levels of serotonin or activation of certain serotonin receptors can cause a condition known as serotonin syndrome, with toxic and potentially fatal effects. In practice, such toxic levels are essentially impossible to reach through an overdose of a single antidepressant drug, but require a combination of serotonergic agents, such as an SSRI with a MAOI, which may occur in therapeutic doses.[153][154] However, serotonin syndrome can occur with overdose of certain serotonin receptor agonists, like the NBOMe series of serotonergic psychedelics.[155][156][157]
The intensity of the symptoms of serotonin syndrome vary over a wide spectrum, and the milder forms are seen even at nontoxic levels.[158] It is estimated that 14% of patients experiencing serotonin syndrome overdose on SSRIs; while the fatality rate is between 2% and 12%.[153][159][160]
Cardiac fibrosis and other fibroses
Some serotonergic agonist drugs cause fibrosis anywhere in the body, particularly the syndrome of retroperitoneal fibrosis, as well as cardiac valve fibrosis.[161]
In the past, three groups of serotonergic drugs have been epidemiologically linked with these syndromes. These are the serotonergic vasoconstrictive antimigraine drugs (ergotamine and methysergide),[161] the serotonergic appetite suppressant drugs (fenfluramine, chlorphentermine, and aminorex), and certain anti-Parkinsonian dopaminergic agonists, which also stimulate serotonergic 5-HT2B receptors. These include pergolide and cabergoline, but not the more dopamine-specific lisuride.[162]
As with fenfluramine, some of these drugs have been withdrawn from the market after groups taking them showed a statistical increase of one or more of the side effects described. An example is pergolide. The drug was declining in use since it was reported in 2003 to be associated with cardiac fibrosis.[163]
Two independent studies published in The New England Journal of Medicine in January 2007 implicated pergolide, along with cabergoline, in causing valvular heart disease.[164][165] As a result of this, the FDA removed pergolide from the United States market in March 2007.[166] (Since cabergoline is not approved in the United States for Parkinson's Disease, but for hyperprolactinemia, the drug remains on the market. Treatment for hyperprolactinemia requires lower doses than that for Parkinson's Disease, diminishing the risk of valvular heart disease).Cite error: Closing </ref> missing for <ref> tag The gastrointestinal parasite Entamoeba histolytica secretes serotonin, causing a sustained secretory diarrhea in some people.[17][167] Patients infected with E. histolytica have been found to have highly elevated serum serotonin levels, which returned to normal following resolution of the infection.[168] E. histolytica also responds to the presence of serotonin by becoming more virulent.[169] This means serotonin secretion not only serves to increase the spread of entamoebas by giving the host diarrhea but also serves to coordinate their behaviour according to their population density, a phenomenon known as quorum sensing. Outside the gut of a host, there is nothing that the entamoebas provoke to release serotonin, hence the serotonin concentration is very low. Low serotonin signals to the entamoebas they are outside a host and they become less virulent to conserve energy. When they enter a new host, they multiply in the gut, and become more virulent as the enterochromaffine cells get provoked by them and the serotonin concentration increases.
Edible plants and mushrooms
In drying seeds, serotonin production is a way to get rid of the buildup of poisonous ammonia. The ammonia is collected and placed in the indole part of L-tryptophan, which is then decarboxylated by tryptophan decarboxylase to give tryptamine, which is then hydroxylated by a cytochrome P450 monooxygenase, yielding serotonin.[170]
However, since serotonin is a major gastrointestinal tract modulator, it may be produced in the fruits of plants as a way of speeding the passage of seeds through the digestive tract, in the same way as many well-known seed and fruit associated laxatives. Serotonin is found in mushrooms, fruits, and vegetables. The highest values of 25–400 mg/kg have been found in nuts of the walnut (Juglans) and hickory (Carya) genera. Serotonin concentrations of 3–30 mg/kg have been found in plantains, pineapples, banana, kiwifruit, plums, and tomatoes. Moderate levels from 0.1–3 mg/kg have been found in a wide range of tested vegetables.[18][15]
Serotonin is one compound of the poison contained in stinging nettles (Urtica dioica), where it causes pain on injection in the same manner as its presence in insect venoms.[16] It is also naturally found in Paramuricea clavata, or the Red Sea Fan.[171]
Serotonin and tryptophan have been found in chocolate with varying cocoa contents. The highest serotonin content (2.93 μg/g) was found in chocolate with 85% cocoa, and the highest tryptophan content (13.27–13.34 μg/g) was found in 70–85% cocoa. The intermediate in the synthesis from tryptophan to serotonin, 5-hydroxytryptophan, was not found.[172]
Root development in Arabidopsis thaliana is stimulated and modulated by serotonin – in various ways at various concentrations.[173]
Serotonin serves as a plant defense chemical against fungi. When infected with Fusarium crown rot (Fusarium pseudograminearum), wheat (Triticum aestivum) greatly increases its production of tryptophan to synthesize new serotonin.[174] The function of this is poorly understood[174] but wheat also produces serotonin when infected by Stagonospora nodorum – in that case to retard spore production.[175] The model cereal Brachypodium distachyon – used as a research substitute for wheat and other production cereals – also produces serotonin, coumaroyl-serotonin, and feruloyl-serotonin in response to F. graminearum. This produces a slight antimicrobial effect. B. distachyon produces more serotonin (and conjugates) in response to deoxynivalenol (DON)-producing F. graminearum than non-DON-producing.[176] Solanum lycopersicum produces many AA conjugates – including several of serotonin – in its leaves, stems, and roots in response to Ralstonia solanacearum infection.[177]
Serotonin occurs in several hallucinogenic mushrooms of the genus Panaeolus.[178]
Invertebrates
Serotonin functions as a neurotransmitter in the nervous systems of most animals.
Nematodes
For example, in the roundworm Caenorhabditis elegans, which feeds on bacteria, serotonin is released as a signal in response to positive events, such as finding a new source of food or in male animals finding a female with which to mate.[179] When a well-fed worm feels bacteria on its cuticle, dopamine is released, which slows it down; if it is starved, serotonin also is released, which slows the animal down further. This mechanism increases the amount of time animals spend in the presence of food.[180] The released serotonin activates the muscles used for feeding, while octopamine suppresses them.[181][182] Serotonin diffuses to serotonin-sensitive neurons, which control the animal's perception of nutrient availability.
Decapods
If lobsters are injected with serotonin, they behave like dominant individuals whereas octopamine causes subordinate behavior.[25] A crayfish that is frightened may flip its tail to flee, and the effect of serotonin on this behavior depends largely on the animal's social status. Serotonin inhibits the fleeing reaction in subordinates, but enhances it in socially dominant or isolated individuals. The reason for this is social experience alters the proportion between serotonin receptors (5-HT receptors) that have opposing effects on the fight-or-flight response.[clarification needed] The effect of 5-HT1 receptors predominates in subordinate animals, while 5-HT2 receptors predominates in dominants.[183]
In venoms
Serotonin is a common component of invertebrate venoms, salivary glands, nervous tissues, and various other tissues, across molluscs, insects, crustaceans, scorpions, various kinds of worms, and jellyfish.[16] Adult Rhodnius prolixus – hematophagous on vertebrates – secrete lipocalins into the wound during feeding. In 2003 these lipocalins were demonstrated to sequester serotonin to prevent vasoconstriction (and possibly coagulation) in the host.[184]
Insects
Serotonin is evolutionarily conserved and appears across the animal kingdom. It is seen in insect processes in roles similar to in the human central nervous system, such as memory, appetite, sleep, and behavior.[185][14] Some circuits in mushroom bodies are serotonergic.[186] (See specific Drosophila example below, §Dipterans.)
Acrididae
Locust swarming is initiated but not maintained by serotonin,[187] with release being triggered by tactile contact between individuals.[188] This transforms social preference from aversion to a gregarious state that enables coherent groups.[189][188][187] Learning in flies and honeybees is affected by the presence of serotonin.[190][191]
Role in insecticides
Insect 5-HT receptors have similar sequences to the vertebrate versions, but pharmacological differences have been seen. Invertebrate drug response has been far less characterized than mammalian pharmacology and the potential for species selective insecticides has been discussed.[192]
Hymenopterans
Wasps and hornets have serotonin in their venom,[193] which causes pain and inflammation[194][16] as do scorpions.[195][16] Pheidole dentata takes on more and more tasks in the colony as it gets older, which requires it to respond to more and more olfactory cues in the course of performing them. This olfactory response broadening was demonstrated to go along with increased serotonin and dopamine, but not octopamine in 2006.[196]
Dipterans
If flies are fed serotonin, they are more aggressive; flies depleted of serotonin still exhibit aggression, but they do so much less frequently.[197] In their crops it plays a vital role in digestive motility produced by contraction. Serotonin that acts on the crop is exogenous to the crop itself and 2012 research suggested that it probably originated in the serotonin neural plexus in the thoracic-abdominal synganglion.[198] In 2011 a Drosophila serotonergic mushroom body was found to work in concert with Amnesiac to form memories.[186] In 2007 serotonin was found to promote aggression in Diptera, which was counteracted by neuropeptide F – a surprising find given that they both promote courtship, which is usually similar to aggression in most respects.[186]
Vertebrates
Serotonin, also referred to as 5-hydroxytryptamine (5-HT), is a neurotransmitter most known for its involvement in mood disorders in humans. It is also a widely present neuromodulator among vertebrates and invertebrates.[199] Serotonin has been found having associations with many physiological systems such as cardiovascular, thermoregulation, and behavioral functions, including: circadian rhythm, appetite, aggressive and sexual behavior, sensorimotor reactivity and learning, and pain sensitivity.[200] Serotonin's function in neurological systems along with specific behaviors among vertebrates found to be strongly associated with serotonin will be further discussed. Two relevant case studies are also mentioned regarding serotonin development involving teleost fish and mice.
In mammals, 5-HT is highly concentrated in the substantia nigra, ventral tegmental area and raphe nuclei. Lesser concentrated areas include other brain regions and the spinal cord.[199] 5-HT neurons are also shown to be highly branched, indicating that they are structurally prominent for influencing multiple areas of the CNS at the same time, although this trend is exclusive solely to mammals.[200]
5-HT system in vertebrates
Vertebrates are multicellular organisms in the phylum Chordata that possess a backbone and a nervous system. This includes mammals, fish, reptiles, birds, etc. In humans, the nervous system is composed of the central and peripheral nervous system, with little known about the specific mechanisms of neurotransmitters in most other vertebrates. However, it is known that while serotonin is involved in stress and behavioral responses, it is also important in cognitive functions.[199] Brain organization in most vertebrates includes 5-HT cells in the hindbrain.[199] In addition to this, 5-HT is often found in other sections of the brain in non-placental vertebrates, including the basal forebrain and pretectum.[201] Since location of serotonin receptors contribute to behavioral responses, this suggests serotonin is part of specific pathways in non-placental vertebrates that are not present in amniotic organisms.[202] Teleost fish and mice are organisms most often used to study the connection between serotonin and vertebrate behavior. Both organisms show similarities in the effect of serotonin on behavior, but differ in the mechanism in which the responses occur.
Dogs / canine species
There are few studies of serotonin in dogs. One study reported serotonin values were higher at dawn than at dusk.[203] In another study, serum 5-HT levels did not seem to be associated with dogs' behavioural response to a stressful situation.[204] Urinary serotonin/creatinine ratio in bitches tended to be higher 4 weeks after surgery. In addition, serotonin was positively correlated with both cortisol and progesterone but not with testosterone after ovariohysterectomy.[205]
Teleost fish
Like non-placental vertebrates, teleost fish also possess 5-HT cells in other sections of the brain, including the basal forebrain.[201] Danio rerio (zebra fish) are a species of teleost fish often used for studying serotonin within the brain. Despite much being unknown about serotonergic systems in vertebrates, the importance in moderating stress and social interaction is known.[206] It is hypothesized that AVT and CRF cooperate with serotonin in the hypothalamic-pituitary-interrenal axis.[201] These neuropeptides influence the plasticity of the teleost, affecting its ability to change and respond to its environment. Subordinate fish in social settings show a drastic increase in 5-HT concentrations.[206] High levels of 5-HT long term influence the inhibition of aggression in subordinate fish.[206]
Mice
Researchers at the Department of Pharmacology and Medical Chemistry used serotonergic drugs on male mice to study the effects of selected drugs on their behavior.[207] Mice in isolation exhibit increased levels of agonistic behavior towards one another. Results found that serotonergic drugs reduce aggression in isolated mice while simultaneously increasing social interaction.[207] Each of the treatments use a different mechanism for targeting aggression, but ultimately all have the same outcome. While the study shows that serotonergic drugs successfully target serotonin receptors, it does not show specifics of the mechanisms that affect behavior, as all types of drugs tended to reduce aggression in isolated male mice.[207] Aggressive mice kept out of isolation may respond differently to changes in serotonin reuptake.
Behavior
Like in humans, serotonin is involved in regulating behavior in most other vertebrates. This includes not only response and social behaviors, but also influencing mood. Defects in serotonin pathways can lead to intense variations in mood, as well as symptoms of mood disorders, which can be present in more than just humans.
Social interaction
One of the most researched aspects of social interaction in which serotonin is involved is aggression. Aggression is regulated by the 5-HT system, as serotonin levels can both induce or inhibit aggressive behaviors, as seen in mice (see section on Mice) and crabs.[207] While this is widely accepted, it is unknown if serotonin interacts directly or indirectly with parts of the brain influencing aggression and other behaviors.[199] Studies of serotonin levels show that they drastically increase and decrease during social interactions, and they generally correlate with inhibiting or inciting aggressive behavior.[208] The exact mechanism of serotonin influencing social behaviors is unknown, as pathways in the 5-HT system in various vertebrates can differ greatly.[199]
Response to stimuli
Serotonin is important in environmental response pathways, along with other neurotransmitters.[209] Specifically, it has been found to be involved in auditory processing in social settings, as primary sensory systems are connected to social interactions.[210] Serotonin is found in the IC structure of the midbrain, which processes specie specific and non-specific social interactions and vocalizations.[210] It also receives acoustic projections that convey signals to auditory processing regions.[210] Research has proposed that serotonin shapes the auditory information being received by the IC and therefore is influential in the responses to auditory stimuli.[210] This can influence how an organism responds to the sounds of predatory or other impactful species in their environment, as serotonin uptake can influence aggression or social interaction.
Mood
We can describe mood not as specific to an emotional status, but as associated with a relatively long-lasting emotional state. Serotonin's association with mood is most known for various forms of depression and bipolar disorders in humans.[200] Disorders caused by serotonergic activity potentially contribute to the many symptoms of major depression, such as overall mood, activity, suicidal thoughts and sexual and cognitive dysfunction. Selective serotonin reuptake inhibitors (SSRI's) are a class of drugs demonstrated to be an effective treatment in major depressive disorder and are the most prescribed class of antidepressants. SSRI's function is to block the reuptake of serotonin, making more serotonin available to absorb by the receiving neuron. Animals have been studied for decades in order to understand depressive behavior among species. One of the most familiar studies, the forced swimming test (FST), was performed to measure potential antidepressant activity.[200] Rats were placed in an inescapable container of water, at which point time spent immobile and number of active behaviors (such as splashing or climbing) were compared before and after a panel of anti-depressant drugs were administered. Antidepressants that selectively inhibit NE reuptake were shown to reduce immobility and selectively increase climbing without affecting swimming. However, results of the SSRI's also show reduced immobility but increased swimming without affecting climbing. This study demonstrated the importance of behavioral tests for antidepressants, as they can detect drugs with an effect on core behavior along with behavioral components of species.[200]
Growth and reproduction
In the nematode C. elegans, artificial depletion of serotonin or the increase of octopamine cues behavior typical of a low-food environment: C. elegans becomes more active, and mating and egg-laying are suppressed, while the opposite occurs if serotonin is increased or octopamine is decreased in this animal.[28] Serotonin is necessary for normal nematode male mating behavior,[211] and the inclination to leave food to search for a mate.[212] The serotonergic signaling used to adapt the worm's behaviour to fast changes in the environment affects insulin-like signaling and the TGF beta signaling pathway,[213] which control long-term adaption.
In the fruit fly insulin both regulates blood sugar as well as acting as a growth factor. Thus, in the fruit fly, serotonergic neurons regulate the adult body size by affecting insulin secretion.[214][215] Serotonin has also been identified as the trigger for swarm behavior in locusts.[189] In humans, though insulin regulates blood sugar and IGF regulates growth, serotonin controls the release of both hormones, modulating insulin release from the beta cells in the pancreas through serotonylation of GTPase signaling proteins.[42] Exposure to SSRIs during pregnancy reduces fetal growth.[216]
Genetically altered C. elegans worms that lack serotonin have an increased reproductive lifespan, may become obese, and sometimes present with arrested development at a dormant larval state.[217][218]
Aging and age-related phenotypes
Serotonin is known to regulate aging, learning, and memory. The first evidence comes from the study of longevity in C. elegans.[213] During early phase of aging , the level of serotonin increases, which alters locomotory behaviors and associative memory.[219] The effect is restored by mutations and drugs (including mianserin and methiothepin) that inhibit serotonin receptors. The observation does not contradict with the notion that the serotonin level goes down in mammals and humans, which is typically seen in late but not early{{vague|date=September 2017}
Biochemical mechanisms
Biosynthesis
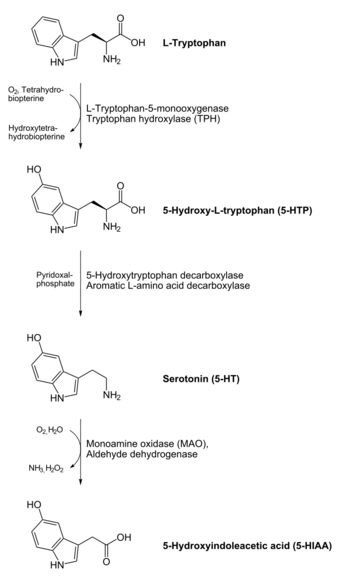
In animals and humans, serotonin is synthesized from the amino acid L-tryptophan by a short metabolic pathway consisting of two enzymes, tryptophan hydroxylase (TPH) and aromatic amino acid decarboxylase (DDC), and the coenzyme pyridoxal phosphate. The TPH-mediated reaction is the rate-limiting step in the pathway.
TPH has been shown to exist in two forms: TPH1, found in several tissues, and TPH2, which is a neuron-specific isoform.[220]
Serotonin can be synthesized from tryptophan in the lab using Aspergillus niger and Psilocybe coprophila as catalysts. The first phase to 5-hydroxytryptophan would require letting tryptophan sit in ethanol and water for 7 days, then mixing in enough HCl (or other acid) to bring the pH to 3, and then adding NaOH to make a pH of 13 for 1 hour. Aspergillus niger would be the catalyst for this first phase. The second phase to synthesizing tryptophan itself from the 5-hydroxytryptophan intermediate would require adding ethanol and water, and letting sit for 30 days this time. The next two steps would be the same as the first phase: adding HCl to make the pH = 3, and then adding NaOH to make the pH very basic at 13 for 1 hour. This phase uses the Psilocybe coprophila as the catalyst for the reaction.[221]
Serotonin taken orally does not pass into the serotonergic pathways of the central nervous system, because it does not cross the blood–brain barrier.[4] However, tryptophan and its metabolite 5-hydroxytryptophan (5-HTP), from which serotonin is synthesized, do cross the blood–brain barrier. These agents are available as dietary supplements and in various foods, and may be effective serotonergic agents.
One product of serotonin breakdown is 5-hydroxyindoleacetic acid (5-HIAA), which is excreted in the urine. Serotonin and 5-HIAA are sometimes produced in excess amounts by certain tumors or cancers, and levels of these substances may be measured in the urine to test for these tumors.
Analytical chemistry
Indium tin oxide is recommended for the electrode material in electrochemical investigation of concentrations produced, detected, or consumed by microbes.[222] A mass spectrometry technique was developed in 1994 to measure the molecular weight of both natural and synthetic serotonins.[223]
History and etymology
It had been known to physiologists for over a century that a vasoconstrictor material appears in serum when blood was allowed to clot.[224] In 1935, Italian Vittorio Erspamer, working in Pavia, showed an extract from enterochromaffin cells made intestines contract. Some believed it contained adrenaline, but two years later, Erspamer was able to show it was a previously unknown amine, which he named "enteramine".[225][226] In 1948, Maurice M. Rapport, Arda Green, and Irvine Page of the Cleveland Clinic discovered a vasoconstrictor substance in blood serum, and since it was a serum agent affecting vascular tone, they named it serotonin.[227]
In 1952, enteramine was shown to be the same substance as serotonin, and as the broad range of physiological roles was elucidated, the abbreviation 5-HT of the proper chemical name 5-hydroxytryptamine became the preferred name in the pharmacological field.[228] Synonyms of serotonin include: 5-hydroxytriptamine, enteramine, substance DS, and 3-(β-aminoethyl)-5-hydroxyindole.[229] In 1953, Betty Twarog and Page discovered serotonin in the central nervous system.[230] Page regarded Erspamer's work on Octopus vulgaris, Discoglossus pictus, Hexaplex trunculus, Bolinus brandaris, Sepia, Mytilus, and Ostrea as valid and fundamental to understanding this newly identified substance, but regarded his earlier results in various models – especially those from rat blood – to be too confounded by the presence of other bioactive chemicals, including some other vasoactives.[231]
Effects in humans
Serotonin, given orally at a dose of 100 mg, produced effects in humans including blood pressure changes, abdominal cramps, muscle aches, and a feeling of sedation.[232][233][234] In contrast to psychedelic drugs like LSD, no hallucinogenic effects were reported.[232][233][234] In other studies, serotonin, at low intravenous doses of 2 to 6 mg, had no effects on electroencephalogram (EEG) readings in humans.[235] In accordance with the preceding findings, it has been stated that administration of serotonin in humans produces no psychoactive effects that cannot be attributed to anxiety by its profound peripheral adverse effects including circulatory disturbance, other autonomic effects, and vomiting.[235][236] Intracerebroventricular injection of serotonin has been studied in patient with severe psychiatric conditions, but little information about its psychoactive effects is provided.[236][237]
It is thought that exogenous serotonin is too hydrophilic to cross the blood–brain barrier and has too poor of metabolic stability due to rapid metabolism by monoamine oxidase (MAO) such that it cannot produce drug-like central effects in humans with peripheral administration.[233][238] However, close analogues of serotonin that are more lipophilic and metabolically stable, like bufotenin (N,N-dimethylserotonin), 5-MeO-DMT (N,N,O-trimethylserotonin), and 5-MeO-AMT (α,O-dimethylserotonin), among many others, are active and produce pronounced centrally mediated effects in humans.[238][239] These drugs are non-selective serotonin receptor agonists like serotonin and are serotonergic psychedelics due to activation of the serotonin 5-HT2A receptor.[238][240][239] α-Methylserotonin is well-studied in preclinical research, but is not known to have been tested in humans.[238]
Notes
- ↑ References for the functions of these receptors are available on the wikipedia pages for the specific receptor in question
References
- ↑ English Pronouncing Dictionary. Cambridge: Cambridge University Press. 2003. ISBN 978-3-12-539683-8.
- ↑ "Serotonin". Dictionary.com Unabridged. Random House. https://www.dictionary.com/browse/Serotonin.
- ↑ "Serotonin". Merriam-Webster Dictionary. https://www.merriam-webster.com/dictionary/Serotonin.
- ↑ 4.0 4.1 "How to increase serotonin in the human brain without drugs". Journal of Psychiatry & Neuroscience 32 (6): 394–399. November 2007. doi:10.1139/jpn.0738. PMID 18043762.
- ↑ "Serotonin differentially modulates excitatory and inhibitory synaptic inputs to putative sleep-promoting neurons of the ventrolateral preoptic nucleus". Neuropharmacology 109: 29–40. October 2016. doi:10.1016/j.neuropharm.2016.05.015. PMID 27238836. https://www.hal.inserm.fr/inserm-02121065v2/file/Serotonin%20differentially%20modulates%20excitatory.pdf.
- ↑ "Serotonin and sleep-promoting neurons". Oncotarget 7 (48): 78222–78223. November 2016. doi:10.18632/oncotarget.13419. PMID 27861160.
- ↑ "Microbes Help Produce Serotonin in Gut". 2015-04-09. https://www.caltech.edu/about/news/microbes-help-produce-serotonin-gut-46495.
- ↑ "Serotonin". The Medical Biochemistry Page. Indiana University School of Medicine. http://themedicalbiochemistrypage.org/nerves.html#5ht.
- ↑ "The expanded biology of serotonin". Annual Review of Medicine 60: 355–366. 2009. doi:10.1146/annurev.med.60.042307.110802. PMID 19630576.
- ↑ "Effect of selective serotonin reuptake inhibitors on platelet activation: can they prevent acute myocardial infarction?". American Journal of Cardiovascular Drugs 3 (3): 149–162. 2003. doi:10.2165/00129784-200303030-00001. PMID 14727927.
- ↑ Kling A (2013). 5-HT2A: a serotonin receptor with a possible role in joint diseases (PDF) (Thesis). Umeå Universitet. ISBN 978-91-7459-549-9.
- ↑ "Serotonin and the vascular wall". International Journal of Cardiology 14 (2): 189–203. February 1987. doi:10.1016/0167-5273(87)90008-8. PMID 3818135.
- ↑ "Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis". Cell 161 (2): 264–276. April 2015. doi:10.1016/j.cell.2015.02.047. PMID 25860609.
- ↑ 14.0 14.1 "The serotonergic central nervous system of the Drosophila larva: anatomy and behavioral function". PLOS ONE 7 (10). 2012. doi:10.1371/journal.pone.0047518. PMID 23082175. Bibcode: 2012PLoSO...747518H.
- ↑ 15.0 15.1 15.2 "Phytoserotonin: a review". Plant Signaling & Behavior 6 (6): 800–809. June 2011. doi:10.4161/psb.6.6.15242. PMID 21617371. Bibcode: 2011PlSiB...6..800A.
- ↑ 16.0 16.1 16.2 16.3 16.4 "Occurrence of indolealkylamines in nature". 5-Hydroxytryptamine and Related Indolealkylamines. Berlin, Heidelberg: Springer Berlin Heidelberg. 1966. pp. 132–181. doi:10.1007/978-3-642-85467-5_4. ISBN 978-3-642-85469-9.
- ↑ 17.0 17.1 "Entamoeba histolytica causes intestinal secretion: role of serotonin". Science 221 (4612): 762–764. August 1983. doi:10.1126/science.6308760. PMID 6308760. Bibcode: 1983Sci...221..762M.
- ↑ 18.0 18.1 "Serotonin content of foods: effect on urinary excretion of 5-hydroxyindoleacetic acid". The American Journal of Clinical Nutrition 42 (4): 639–643. October 1985. doi:10.1093/ajcn/42.4.639. PMID 2413754.
- ↑ "Ingestion of Japanese plums (Prunus salicina Lindl. cv. Crimson Globe) increases the urinary 6-sulfatoxymelatonin and total antioxidant capacity levels in young, middle-aged and elderly humans: Nutritional and functional characterization of their content". Journal of Food and Nutrition Research 50 (4): 229–236. 2011. https://www.researchgate.net/publication/259983119.
- ↑ "Towards an understanding of crystallization from solution. DFT studies of multi-component serotonin crystals" (in en). Computational and Theoretical Chemistry 1088: 52–61. July 2016. doi:10.1016/j.comptc.2016.04.027.
- ↑ "Crystal structure of serotonin". Acta Crystallographica Section E 78 (Pt 4): 365–368. April 2022. doi:10.1107/S2056989022002559. PMID 35492269. Bibcode: 2022AcCrE..78..365N.
- ↑ "A new structure of a serotonin salt: comparison and conformational analysis of all known serotonin complexes". Acta Crystallographica Section C 69 (Pt 9): 1055–1061. September 2013. doi:10.1107/S0108270113019823. PMID 24005521. Bibcode: 2013AcCrC..69.1055R.
- ↑ "Serotonin: a review". Journal of Veterinary Pharmacology and Therapeutics 31 (3): 187–199. June 2008. doi:10.1111/j.1365-2885.2008.00944.x. PMID 18471139.
- ↑ "Modulating role of serotonergic signaling in sleep and memory". Pharmacological Reports 74 (1): 1–26. February 2022. doi:10.1007/s43440-021-00339-8. PMID 34743316.
- ↑ 25.0 25.1 "Hormonal control of behavior: amines and the biasing of behavioral output in lobsters". Science 241 (4874): 1775–1781. September 1988. doi:10.1126/science.2902685. PMID 2902685. Bibcode: 1988Sci...241.1775K.
- ↑ "Plasticity of thalamocortical axons is regulated by serotonin levels modulated by preterm birth". Proceedings of the National Academy of Sciences of the United States of America 120 (33). August 2023. doi:10.1073/pnas.2301644120. PMID 37549297. Bibcode: 2023PNAS..12001644S.
- ↑ 27.0 27.1 "5-Hydroxytryptamine receptors". Pharmacological Reviews 44 (3): 401–458. September 1992. doi:10.1016/S0031-6997(25)00462-4. PMID 1359584.
- ↑ 28.0 28.1 "Serotonin regulates C. elegans fat and feeding through independent molecular mechanisms". Cell Metabolism 7 (6): 533–544. June 2008. doi:10.1016/j.cmet.2008.04.012. PMID 18522834.
- ↑ "Influence of abiotic stress signals on secondary metabolites in plants". Plant Signaling & Behavior (Informa) 6 (11): 1720–1731. November 2011. doi:10.4161/psb.6.11.17613. PMID 22041989. Bibcode: 2011PlSiB...6.1720A.
- ↑ Psychiatry Under the Influence: Institutional Corruption, Social Injury, and Prescriptions for Reform. Springer. 2015. pp. 55–56. ISBN 978-1-137-51602-2. https://books.google.com/books?id=fxPACQAAQBAJ&pg=PA55.
- ↑ "The serotonin theory of depression: a systematic umbrella review of the evidence". Molecular Psychiatry (Nature Publishing Group) 28 (8): 3243–3256. August 2023. doi:10.1038/s41380-022-01661-0. PMID 35854107.
- ↑ Has the Serotonin Hypothesis Been Debunked?, 2022, https://www.psychologytoday.com/us/blog/mood-swings/202210/has-the-serotonin-hypothesis-been-debunked, retrieved 2 May 2023
- ↑ "The Degradation of Serotonin: Role of MAO". Handbook of Behavioral Neuroscience. 21. Elsevier. 2010. pp. 203–218. doi:10.1016/s1569-7339(10)70079-5. ISBN 978-0-12-374634-4.
- ↑ "Tryptophan hydroxylase as novel target for the treatment of depressive disorders". Pharmacology 85 (2): 95–109. 2010. doi:10.1159/000279322. PMID 20130443.
- ↑ "How Monoamine Oxidase A Decomposes Serotonin: An Empirical Valence Bond Simulation of the Reactive Step". The Journal of Physical Chemistry B 124 (38): 8259–8265. September 2020. doi:10.1021/acs.jpcb.0c06502. PMID 32845149.
- ↑ "Molecular biology of 5-HT receptors". Behavioural Brain Research 195 (1): 198–213. December 2008. doi:10.1016/j.bbr.2008.03.020. PMID 18571247.
- ↑ 37.0 37.1 "Trace Amines and Their Receptors". Pharmacol Rev 70 (3): 549–620. July 2018. doi:10.1124/pr.117.015305. PMID 29941461.
- ↑ 38.0 38.1 "In Vitro Characterization of Psychoactive Substances at Rat, Mouse, and Human Trace Amine-Associated Receptor 1". J Pharmacol Exp Ther 357 (1): 134–144. April 2016. doi:10.1124/jpet.115.229765. PMID 26791601. https://d1wqtxts1xzle7.cloudfront.net/74120533/eae6c6e62565b82d46b4d111bbea0f77b9c2-libre.pdf?1635931703=&response-content-disposition=inline%3B+filename%3DIn_Vitro_Characterization_of_Psychoactiv.pdf&Expires=1746838268&Signature=Sy4fJ90yUhxs68314NxYsW5PAaNrBGePRu35WRR4PIF-3YC7Z~sLdnCn5wfqqbLg9bDEGdt~oW55ugMP3D3jgA0BoRI~~GOb0NQOwrtfUEQK1PQs1uuN9qg5Y1ct8z5NsABm44RgtukkwRMdU6fO7OlfIsQ68hOiFk129Ll7UYqldxD2f1xhE2fTTfsxSpb8cMCJzHn7-ItqLdwnAUPFK7WggDIjmY1kCnaHLwIxMwdJCAq8L6DYzSTg7pZkbR8qlou~GXbTPQt~gYpyZTJp5hgW-7V6K5wLlQ7Z2xE7B0f9wEfuc1W1QNafg125Tr-vvAe4LEGKXV58bnn1bpfWKw__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA.
- ↑ "The structural diversity of psychedelic drug actions revealed". Nature Communications 16 (1). March 2025. doi:10.1038/s41467-025-57956-7. PMID 40108183. Bibcode: 2025NatCo..16.2734G.
- ↑ "Structures of Hallucinogenic and Non-Hallucinogenic Analogues of the 5-HT2A Receptor Reveals Molecular Insights into Signaling Bias". University of North Carolina at Chapel Hill Department of Pharmacology Research Retreat September 16th, 2022 – William and Ida Friday Center. September 2022. https://www.med.unc.edu/pharm/wp-content/uploads/sites/930/2022/07/COMPLETE-PHARM-RETREAT-PROGRAM-2022-UPDATE.pdf#page=37.
- ↑ 41.0 41.1 "Evidence for significant contribution of a newly identified monoamine transporter (PMAT) to serotonin uptake in the human brain". Biochemical Pharmacology 73 (1): 147–154. January 2007. doi:10.1016/j.bcp.2006.09.008. PMID 17046718.
- ↑ 42.0 42.1 42.2 "Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation". PLOS Biology 7 (10). October 2009. doi:10.1371/journal.pbio.1000229. PMID 19859528.
- ↑ "Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release". Cell 115 (7): 851–862. December 2003. doi:10.1016/S0092-8674(03)01014-6. PMID 14697203.
- ↑ "Serotonylation of vascular proteins important to contraction". PLOS ONE 4 (5). May 2009. doi:10.1371/journal.pone.0005682. PMID 19479059. Bibcode: 2009PLoSO...4.5682W.
- ↑ "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. 12 January 2011. http://pdsp.med.unc.edu/pdsp.php.
- ↑ "The activation of 5-HT receptors in prefrontal cortex enhances dopaminergic activity". Journal of Neurochemistry 95 (6): 1597–1607. December 2005. doi:10.1111/j.1471-4159.2005.03485.x. PMID 16277612.
- ↑ "5-HT2A receptor enhancement of contractile activity of the porcine urothelium and lamina propria". International Journal of Urology 23 (11): 946–951. November 2016. doi:10.1111/iju.13172. PMID 27531585.
- ↑ "Von Economo neuron – NeuronBank". http://neuronbank.org/wiki/index.php/Von_Economo_neuron.[unreliable medical source?]
- ↑ "The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways". The Journal of Pharmacology and Experimental Therapeutics 306 (3): 954–964. September 2003. doi:10.1124/jpet.103.051797. PMID 12750432.
- ↑ "Role of 5-HT5A receptors in the consolidation of memory". Behavioural Brain Research 252: 246–251. September 2013. doi:10.1016/j.bbr.2013.05.051. PMID 23735322.
- ↑ "Serotonin receptors in depression: from A to B". F1000Research 6: 123. 2017. doi:10.12688/f1000research.9736.1. PMID 28232871.
- ↑ "Understanding the neuroanatomical organization of serotonergic cells in the brain provides insight into the functions of this neurotransmitter". Basic Neurochemistry (Sixth ed.). Lippincott Williams & Wilkins. 1999. ISBN 978-0-397-51820-3. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Search&db=books&doptcmdl=GenBookHL&term=raphe+AND+serotonin+release+AND+bnchm%5Bbook%5D+AND+160428%5Buid%5D&rid=bnchm.section.946#949. "In 1964, Dahlstrom and Fuxe (discussed in [2]), using the Falck-Hillarp technique of histofluorescence, observed that the majority of serotonergic soma are found in cell body groups, which previously had been designated as the Raphe nuclei."
- ↑ encyclopedia of neuroscience. Berlin: Springer. 2009. p. 705. ISBN 978-3-540-23735-8.
- ↑ The raphe nuclei group of neurons are located along the brain stem from the labels 'Mid Brain' to 'Oblongata', centered on the pons. (See relevant image.)
- ↑ "Organization of Functional Long-Range Circuits Controlling the Activity of Serotonergic Neurons in the Dorsal Raphe Nucleus". Cell Reports 18 (12): 3018–3032. March 2017. doi:10.1016/j.celrep.2017.02.077. PMID 28329692.
- ↑ "A prospective code for value in the serotonin system". Nature 641 (8064): 952–959. March 2025. doi:10.1038/s41586-025-08731-7. PMID 40140568.
- ↑ Handbook of the behavioral neurobiology of serotonin (1st ed.). London: Academic. 2009. pp. 51–59. ISBN 978-0-12-374634-4.
- ↑ "Regulation of the Serotonergic System by Kainate in the Avian Retina". Cellular and Molecular Neurobiology 39 (7): 1039–1049. October 2019. doi:10.1007/s10571-019-00701-8. PMID 31197744.
- ↑ "Serotonin in Pain and Pain Control". Handbook of the behavioral neurobiology of serotonin (1st ed.). London: Academic. 2009. pp. 457–460. ISBN 978-0-12-374634-4.
- ↑ "Serotonin in Mode and Emotions". Handbook of the behavioral neurobiology of serotonin (1st ed.). London: Academic. 2009. pp. 367–399. ISBN 978-0-12-374634-4.
- ↑ "Is serotonin an upper or a downer? The evolution of the serotonergic system and its role in depression and the antidepressant response". Neuroscience and Biobehavioral Reviews 51: 164–188. April 2015. doi:10.1016/j.neubiorev.2015.01.018. PMID 25625874.
- ↑ "Which comes first: atypical antipsychotic treatment or cardiometabolic risk?". Acta Psychiatrica Scandinavica 119 (3): 171–179. March 2009. doi:10.1111/j.1600-0447.2008.01334.x. PMID 19178394.
- ↑ "Low gene expression conferred by association of an allele of the 5-HT2C receptor gene with antipsychotic-induced weight gain". The American Journal of Psychiatry 162 (3): 613–615. March 2005. doi:10.1176/appi.ajp.162.3.613. PMID 15741483.
- ↑ "Dysregulation of diurnal rhythms of serotonin 5-HT2C and corticosteroid receptor gene expression in the hippocampus with food restriction and glucocorticoids". The Journal of Neuroscience 17 (11): 4056–4065. June 1997. doi:10.1523/JNEUROSCI.17-11-04056.1997. PMID 9151722.
- ↑ "The role of serotonin in eating disorders". Drugs 39 (Suppl 3): 33–48. 1990. doi:10.2165/00003495-199000393-00005. PMID 2197074.
- ↑ McGuire, Michael (2013) "Believing, the neuroscience of fantasies, fears, and confictions" (Prometius Books)
- ↑ "Pindolol augmentation in aggressive schizophrenic patients: a double-blind crossover randomized study". International Clinical Psychopharmacology 16 (2): 111–115. March 2001. doi:10.1097/00004850-200103000-00006. PMID 11236069.
- ↑ "[Proceedings: Study of gastrointestinal motility using an extraluminal force transducer. 6. Observation of gastric and duodenal motility using synthetic motilin]". Nihon Heikatsukin Gakkai Zasshi 11 (4): 244–246. December 1975. PMID 1232434.
- ↑ "Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region". Science 274 (5292): 1527–1531. November 1996. doi:10.1126/science.274.5292.1527. PMID 8929413. Bibcode: 1996Sci...274.1527L.
- ↑ "Serotonin pharmacology in the gastrointestinal tract: a review". Naunyn-Schmiedeberg's Archives of Pharmacology 377 (3): 181–203. May 2008. doi:10.1007/s00210-008-0276-9. PMID 18398601.
- ↑ Pharmacology. Edinburgh: Churchill Livingstone. 2003. p. 187. ISBN 978-0-443-07145-4.
- ↑ "Is there a pharmacological basis for differences in 5-HT3-receptor antagonist efficacy in refractory patients?". Cancer Chemotherapy and Pharmacology 56 (3): 231–238. September 2005. doi:10.1007/s00280-005-1033-0. PMID 15838653.
- ↑ 73.0 73.1 "Serotonin producing neuroepithelial bodies in rabbit respiratory mucosa". Science 180 (4084): 410–413. April 1973. doi:10.1126/science.180.4084.410. PMID 4121716. Bibcode: 1973Sci...180..410L.
- ↑ "Immunocytochemical localization of serotonin in the reptilian lung". Cell and Tissue Research (Springer Science and Business Media LLC) 248 (3): 713–715. June 1987. doi:10.1007/bf00216504. PMID 3301000.
- ↑ 75.0 75.1 "Morphological and cytochemical characterization of neuroepithelial bodies in fetal rabbit lung. I. Studies of isolated neuroepithelial bodies". Experimental Lung Research 3 (3–4): 349–377. November 1982. doi:10.3109/01902148209069663. PMID 6132813.
- ↑ "Merkel disc is a serotonergic synapse in the epidermis for transmitting tactile signals in mammals". Proceedings of the National Academy of Sciences of the United States of America 113 (37): E5491–E5500. September 2016. doi:10.1073/pnas.1610176113. PMID 27573850. Bibcode: 2016PNAS..113E5491C.
- ↑ "Patients with high-bone-mass phenotype owing to Lrp5-T253I mutation have low plasma levels of serotonin". Journal of Bone and Mineral Research 25 (3): 673–675. March 2010. doi:10.1002/jbmr.44. PMID 20200960.
- ↑ "Breaking into bone biology: serotonin's secrets". Nature Medicine 15 (2): 145–146. February 2009. doi:10.1038/nm0209-145. PMID 19197289.
- ↑ "Relation of serum serotonin levels to bone density and structural parameters in women". Journal of Bone and Mineral Research 25 (2): 415–422. February 2010. doi:10.1359/jbmr.090721. PMID 19594297.
- ↑ "Levels of serotonin, sclerostin, bone turnover markers as well as bone density and microarchitecture in patients with high-bone-mass phenotype due to a mutation in Lrp5". Journal of Bone and Mineral Research 26 (8): 1721–1728. August 2011. doi:10.1002/jbmr.376. PMID 21351148.
- ↑ "FOXO1 orchestrates the bone-suppressing function of gut-derived serotonin". The Journal of Clinical Investigation 122 (10): 3490–3503. October 2012. doi:10.1172/JCI64906. PMID 22945629.
- ↑ "Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis". Nature Medicine 16 (3): 308–312. March 2010. doi:10.1038/nm.2098. PMID 20139991.
- ↑ "RNA Sensing by Gut Piezo1 Is Essential for Systemic Serotonin Synthesis". Cell 182 (3): 609–624.e21. August 2020. doi:10.1016/j.cell.2020.06.022. PMID 32640190.
- ↑ "Lifespan: catch-up growth and obesity in male mice". Nature 427 (6973): 411–412. January 2004. doi:10.1038/427411b. PMID 14749819. Bibcode: 2004Natur.427..411O.
- ↑ "Preweaning food intake influences the adiposity of young adult baboons". The Journal of Clinical Investigation 78 (4): 899–905. October 1986. doi:10.1172/JCI112678. PMID 3760191.
- ↑ "Effect of litter size on plasma cholesterol and insulin and some liver and adipose tissue enzymes in adult rodents". The Journal of Nutrition 114 (7): 1231–1234. July 1984. doi:10.1093/jn/114.7.1231. PMID 6376732.
- ↑ "Lasting syndrome of depression produced by reduction in serotonin uptake during postnatal development: evidence from sleep, stress, and behavior". The Journal of Neuroscience 28 (14): 3546–3554. April 2008. doi:10.1523/JNEUROSCI.4006-07.2008. PMID 18385313.
- ↑ "Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry". Neuropsychopharmacology 31 (1): 47–57. January 2006. doi:10.1038/sj.npp.1300823. PMID 16012532.
- ↑ "Neonatal citalopram exposure produces lasting changes in behavior which are reversed by adult imipramine treatment". European Journal of Pharmacology 532 (3): 265–269. February 2006. doi:10.1016/j.ejphar.2005.12.081. PMID 16483567.
- ↑ "Neuroscience. Prozac treatment of newborn mice raises anxiety". Science 306 (5697): 792. October 2004. doi:10.1126/science.306.5697.792. PMID 15514122.
- ↑ "Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice". Science 306 (5697): 879–881. October 2004. doi:10.1126/science.1101678. PMID 15514160. Bibcode: 2004Sci...306..879A.
- ↑ "Platelet-derived serotonin mediates liver regeneration". Science 312 (5770): 104–107. April 2006. doi:10.1126/science.1123842. PMID 16601191. Bibcode: 2006Sci...312..104L.
- ↑ "Deletion of the serotonin transporter in rats disturbs serotonin homeostasis without impairing liver regeneration". American Journal of Physiology. Gastrointestinal and Liver Physiology 296 (4): G963–G968. April 2009. doi:10.1152/ajpgi.90709.2008. PMID 19246633. http://www.suaire.suanet.ac.tz:8080/xmlui/handle/123456789/2619. Retrieved 5 December 2019.
- ↑ "The serotonin 5-HT2B receptor controls bone mass via osteoblast recruitment and proliferation". FASEB Journal 22 (2): 418–427. February 2008. doi:10.1096/fj.07-9209com. PMID 17846081.
- ↑ "Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum". Cell 135 (5): 825–837. November 2008. doi:10.1016/j.cell.2008.09.059. PMID 19041748.
- "It Takes Guts To Build Bone, Scientists Discover". ScienceDaily (Press release). December 1, 2008.
- ↑ "5-hydroxytryptamine stimulates phosphorylation of p44/p42 mitogen-activated protein kinase activation in bovine aortic endothelial cell cultures". Journal of Cardiovascular Pharmacology 35 (3): 398–402. March 2000. doi:10.1097/00005344-200003000-00008. PMID 10710124.
- ↑ "Pulmonary neuroendocrine cells: physiology, tissue homeostasis and disease". Disease Models & Mechanisms 13 (12). December 2020. doi:10.1242/dmm.046920. PMID 33355253.
- ↑ "Regulation of systemic energy homeostasis by serotonin in adipose tissues". Nature Communications 6. April 2015. doi:10.1038/ncomms7794. PMID 25864946. Bibcode: 2015NatCo...6.6794O.
- ↑ "Pharmacology of central serotonin neurons". Annual Review of Pharmacology and Toxicology 20: 111–127. 1980. doi:10.1146/annurev.pa.20.040180.000551. PMID 6992697.
- ↑ Goodman and Gilman's pharmacological basis of therapeutics. New York: McGraw-Hill. 2001. pp. 459–461. ISBN 978-0-07-162442-8.
- ↑ "Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level". The Journal of Neuroscience 19 (23): 10494–10501. December 1999. doi:10.1523/JNEUROSCI.19-23-10494.1999. PMID 10575045.
- ↑ "Serotonin transporter polymorphisms and persistent, pervasive childhood aggression". The American Journal of Psychiatry 163 (6): 1103–1105. June 2006. doi:10.1176/appi.ajp.163.6.1103. PMID 16741214.
- ↑ "5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression". Nature Neuroscience 8 (6): 828–834. June 2005. doi:10.1038/nn1463. PMID 15880108.
- ↑ "A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety". Molecular Psychiatry 9 (2): 197–202. February 2004. doi:10.1038/sj.mp.4001405. PMID 14966478.
- ↑ "Antidepressant therapy for unexplained symptoms and symptom syndromes". J Fam Pract 48 (12): 980–990. December 1999. PMID 10628579.
- ↑ "Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia". Cochrane Database Syst Rev 2 (2). February 2018. doi:10.1002/14651858.CD010292.pub2. PMID 29489029.
- ↑ "Buspirone and related compounds as alternative anxiolytics". Neuropeptides 19 (Suppl): 15–19. July 1991. doi:10.1016/0143-4179(91)90078-w. PMID 1679210.
- ↑ "Azapirone 5-HT1A receptor partial agonist treatment for major depressive disorder: systematic review and meta-analysis". Psychol Med 44 (11): 2255–2269. August 2014. doi:10.1017/S0033291713002857. PMID 24262766.
- ↑ 109.0 109.1 "The role of serotonin in antipsychotic drug action". Neuropsychopharmacology 21 (2 Suppl): 106S–115S. August 1999. doi:10.1016/S0893-133X(99)00046-9. PMID 10432496.
- ↑ 110.0 110.1 "Serotonergic Mechanisms as Targets for Existing and Novel Antipsychotics". Handb Exp Pharmacol. Handbook of Experimental Pharmacology. 212. 2012. pp. 87–124. doi:10.1007/978-3-642-25761-2_4. ISBN 978-3-642-25760-5.
- ↑ "Triptans in migraine: a comparative review of pharmacology, pharmacokinetics and efficacy". Drugs 60 (6): 1259–1287. December 2000. doi:10.2165/00003495-200060060-00003. PMID 11152011.
- ↑ 112.0 112.1 "Activation of 5-hydroxytryptamine1B/1D/1F receptors as a mechanism of action of antimigraine drugs". Expert Opin Pharmacother 14 (12): 1599–1610. August 2013. doi:10.1517/14656566.2013.806487. PMID 23815106.
- ↑ "Pharmacology of antimigraine drugs". J Neurol 238 (Suppl 1): S28–S35. 1991. doi:10.1007/BF01642903. PMID 1646288.
- ↑ "The 5-HT1B and 5-HT1D agonists in acute migraine therapy: Ergotamine, dihydroergotamine, and the triptans". Handbook of Clinical Neurology. 199. 2024. pp. 17–42. doi:10.1016/B978-0-12-823357-3.00008-2. ISBN 978-0-12-823357-3.
- ↑ 115.0 115.1 "Ergot and its alkaloids". American Journal of Pharmaceutical Education 70 (5): 98. October 2006. doi:10.5688/aj700598. PMID 17149427. "Ergonovine is a selective and moderately potent tryptaminergic receptor antagonist in various smooth muscles, being only a partially agonistic or antagonistic at tryptaminergic receptors in the central nervous system. In blood vessels the alkaloid is only weakly antagonistic of dopaminergic receptors and partitally agonistic of α-adrenergic receptors. The most pronounced effect of ergonovine is one of direct stimulation of the uterine smooth musculature, resulting in increased muscular tone and an enhancement of the rate and force of rhythmical contractions. This stimulant effect seems to be most closely associated with agonist or partial agonist effects at 5-HT2 receptors. [...] LSD and related hallucinogens are known to interact with brain 5-HT receptors to produce agonist or partial antagonist effects on serotonin activity.".
- ↑ 116.0 116.1 "Uterotonic Medications: Oxytocin, Methylergonovine, Carboprost, Misoprostol". Anesthesiology Clinics 35 (2): 207–219. June 2017. doi:10.1016/j.anclin.2017.01.007. PMID 28526143. "Methylergonovine is a serotonergic receptor agonist in the smooth muscle. It is also a weak antagonist of dopaminergic receptors and partial agonist of α-adrenergic receptors.22 Methylergonovine causes uterine contractions and relaxation at low doses, but causes sustained contractions and increased basal tone at high doses.24 The mechanism of action for uterine contraction is not well defined. Uterine contraction is likely produced by methylergonovine agonist effects on the 5-HT2 receptor found in uterine smooth muscle.22 Alternatively, methylergonovine could cause uterine contraction through direct stimulation of the α-adrenergic receptors in the uterus, which has been postulated to lead to calcium mobilization.25".
- ↑ 117.0 117.1 "Pharmacology, pharmacogenetics, and clinical efficacy of 5-hydroxytryptamine type 3 receptor antagonists for postoperative nausea and vomiting". Curr Opin Anesthesiol 19 (6): 606–611. December 2006. doi:10.1097/01.aco.0000247340.61815.38. PMID 17093363.
- ↑ 118.0 118.1 "5-HT3 receptor antagonists, a new approach in emesis: a review of ondansetron, granisetron and tropisetron". Anticancer Drugs 2 (4): 343–355. August 1991. doi:10.1097/00001813-199108000-00003. PMID 1665723.
- ↑ "Serotonergic drugs: effects on appetite expression and use for the treatment of obesity". Drugs 67 (1): 27–55. 2007. doi:10.2165/00003495-200767010-00004. PMID 17209663.
- ↑ "Lorcaserin: an investigational serotonin 2C agonist for weight loss". Am J Health Syst Pharm 68 (21): 2029–2037. November 2011. doi:10.2146/ajhp100638. PMID 22011982.
- ↑ "Serotonergic anti-obesity agents: past experience and future prospects". Drugs 71 (17): 2247–2255. December 2011. doi:10.2165/11596680-000000000-00000. PMID 22085383.
- ↑ "5-HT2C Receptor Agonists and the Control of Appetite". Handbook of Experimental Pharmacology. 209. 2012. pp. 349–356. doi:10.1007/978-3-642-24716-3_16. ISBN 978-3-642-24715-6.
- ↑ 123.0 123.1 "5-HT2C Receptor Stimulation in Obesity Treatment: Orthosteric Agonists vs. Allosteric Modulators". Nutrients 15 (6): 1449. March 2023. doi:10.3390/nu15061449. PMID 36986191.
- ↑ 124.0 124.1 "Reintroducing Fenfluramine as a Treatment for Seizures: Current Knowledge, Recommendations and Gaps in Understanding". Neuropsychiatr Dis Treat 19: 2013–2025. 2023. doi:10.2147/NDT.S417676. PMID 37790801.
- ↑ "Lorcaserin for Dravet Syndrome: A Potential Advance Over Fenfluramine?". CNS Drugs 36 (2): 113–122. February 2022. doi:10.1007/s40263-022-00896-3. PMID 35094259.
- ↑ "Evaluating bexicaserin for the treatment of developmental epileptic encephalopathies". Expert Opin Pharmacother 25 (9): 1121–1130. June 2024. doi:10.1080/14656566.2024.2373350. PMID 38916481.
- ↑ "BMB 101". 23 October 2024. https://adisinsight.springer.com/drugs/800065004.
- ↑ 128.0 128.1 "Molecular insights into psychedelic drug action". J Neurochem 162 (1): 24–38. July 2022. doi:10.1111/jnc.15540. PMID 34797943.
- ↑ 129.0 129.1 "Serotonin 2A Receptor (5-HT2AR) Agonists: Psychedelics and Non-Hallucinogenic Analogues as Emerging Antidepressants". Chem Rev 124 (1): 124–163. January 2024. doi:10.1021/acs.chemrev.3c00375. PMID 38033123.
- ↑ 130.0 130.1 Chemistry and Structure-Activity Relationships of Psychedelics. Current Topics in Behavioral Neurosciences. 36. 2018. pp. 1–43. doi:10.1007/7854_2017_475. ISBN 978-3-662-55878-2.
- ↑ "Drug-drug interactions involving classic psychedelics: A systematic review". J Psychopharmacol 38 (1): 3–18. January 2024. doi:10.1177/02698811231211219. PMID 37982394.
- ↑ 132.00 132.01 132.02 132.03 132.04 132.05 132.06 132.07 132.08 132.09 "Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors". Science 379 (6633): 700–706. February 2023. doi:10.1126/science.adf0435. PMID 36795823. Bibcode: 2023Sci...379..700V. "In addition to promoting psychedelic-induced structural neuroplasticity, the intracellular population of 5-HT2ARs might also contribute to the hallucinogenic effects of psychedelics. When we administered a serotonin-releasing agent to wild type mice, we did not observe a HTR. However, the same drug was able to induce a HTR in mice expressing SERT on cortical neurons of the mPFC—a brain region known to be essential for the HTR (49). Thus, activation of intracellular cortical 5-HT2ARs may play a role in the subjective effects of psychedelics. This hypothesis is further supported by previous work demonstrating that a high dose of the serotonin precursor 5-hydroxytryptophan (5-HTP) induces a HTR in WT mice, which can be blocked by an N-methyltransferase inhibitor that prevents the metabolism of 5-HTP to N-methyltryptamines (50). Inhibition of N-methyltransferase failed to block the HTR induced by 5-MeO-DMT (50). Taken together, this work emphasizes that accessing intracellular 5-HT2ARs is important for 5-HT2AR agonists to produce a HTR.".
- ↑ 133.0 133.1 133.2 133.3 133.4 133.5 133.6 133.7 "The Key Role of Intracellular 5-HT2A Receptors: A Turning Point in Psychedelic Research?". Psychoactives 2 (4): 287–293. 13 October 2023. doi:10.3390/psychoactives2040018. ISSN 2813-1851.
- ↑ "βArrestins: Ligand-Directed Regulators of 5-HT2A Receptor Trafficking and Signaling Events". 5-HT2A Receptors in the Central Nervous System. Cham: Springer International Publishing. 2018. pp. 31–55. doi:10.1007/978-3-319-70474-6_2. ISBN 978-3-319-70472-2.
- ↑ 135.0 135.1 135.2 "Animal Models and Hallucinogenic Drugs". The Neuroscience of Hallucinations. New York, NY: Springer New York. 2013. pp. 253–277. doi:10.1007/978-1-4614-4121-2_14. ISBN 978-1-4614-4120-5.
- ↑ 136.0 136.1 136.2 "Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a β-arrestin2/Src/Akt signaling complex in vivo". J Neurosci 30 (40): 13513–24. October 2010. doi:10.1523/JNEUROSCI.1665-10.2010. PMID 20926677.
- ↑ "Effect of Hallucinogens on Unconditioned Behavior". Behavioral Neurobiology of Psychedelic Drugs. Current Topics in Behavioral Neurosciences. 36. 2018. pp. 159–199. doi:10.1007/7854_2016_466. ISBN 978-3-662-55878-2.
- ↑ "Is 5-methoxytryptamine a pineal hormone?". Psychoneuroendocrinology 8 (1): 61–73. 1983. doi:10.1016/0306-4530(83)90041-0. PMID 6136058.
- ↑ "On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: comparisons across species". J Pineal Res 61 (1): 27–40. August 2016. doi:10.1111/jpi.12336. PMID 27112772.
- ↑ "5-Methoxytryptamine-induced head twitches in rats". Pol J Pharmacol Pharm 29 (3): 253–261. 1977. PMID 267911.
- ↑ "Significance of mammalian N, N-dimethyltryptamine (DMT): A 60-year-old debate". J Psychopharmacol 36 (8): 905–919. August 2022. doi:10.1177/02698811221104054. PMID 35695604.
- ↑ "N, N-Dimethyltryptamine (DMT), an Endogenous Hallucinogen: Past, Present, and Future Research to Determine Its Role and Function". Front Neurosci 12. 2018. doi:10.3389/fnins.2018.00536. PMID 30127713.
- ↑ "Dark Classics in Chemical Neuroscience: N, N-Dimethyltryptamine (DMT)". ACS Chem Neurosci 9 (10): 2344–2357. October 2018. doi:10.1021/acschemneuro.8b00101. PMID 30036036.
- ↑ 144.0 144.1 "Dark Classics in Chemical Neuroscience: 3,4-Methylenedioxymethamphetamine". ACS Chem Neurosci 9 (10): 2408–2427. October 2018. doi:10.1021/acschemneuro.8b00155. PMID 30001118.
- ↑ 145.0 145.1 "Entactogens: How the Name for a Novel Class of Psychoactive Agents Originated". Front Psychiatry 13. 2022. doi:10.3389/fpsyt.2022.863088. PMID 35401275.
- ↑ "Drug-drug interactions between psychiatric medications and MDMA or psilocybin: a systematic review". Psychopharmacology (Berl) 239 (6): 1945–1976. June 2022. doi:10.1007/s00213-022-06083-y. PMID 35253070.
- ↑ "Therapeutic potential of monoamine transporter substrates". Curr Top Med Chem 6 (17): 1845–1859. 2006. doi:10.2174/156802606778249766. PMID 17017961.
- ↑ "Effects of 5-HT-releasing agents on the extracellullar hippocampal 5-HT of rats. Implications for the development of novel antidepressants with a short onset of action". Neuropharmacology 38 (7): 1055–1061. July 1999. doi:10.1016/S0028-3908(99)00023-4. PMID 10428424.
- ↑ "The Effect of Selective Serotonin Releasing Agents in the Chronic Mild Stress Model of Depression in Rats". Stress 2 (2): 91–100. December 1997. doi:10.3109/10253899709014740. PMID 9787258.
- ↑ "Drug discrimination studies of the interoceptive cues produced by selective serotonin uptake inhibitors and selective serotonin releasing agents". Psychopharmacology (Berl) 138 (1): 67–75. July 1998. doi:10.1007/s002130050646. PMID 9694528.
- ↑ "Pharmacology of MDMA- and Amphetamine-Like New Psychoactive Substances". Handb Exp Pharmacol. Handbook of Experimental Pharmacology 252: 143–164. 2018. doi:10.1007/164_2018_113. ISBN 978-3-030-10560-0. PMID 29633178.
- ↑ "Beyond ecstasy: Alternative entactogens to 3,4-methylenedioxymethamphetamine with potential applications in psychotherapy". J Psychopharmacol 35 (5): 512–536. May 2021. doi:10.1177/0269881120920420. PMID 32909493.
- ↑ 153.0 153.1 "Psychiatric Emergencies in the Intensive Care Unit". AACN Advanced Critical Care 26 (4): 285–293; quiz 294–295. 2015-10-01. doi:10.4037/NCI.0000000000000104. PMID 26484986.
- ↑ "Relative toxicity of selective serotonin reuptake inhibitors (SSRIs) in overdose". Journal of Toxicology. Clinical Toxicology 42 (3): 277–285. 2004. doi:10.1081/CLT-120037428. PMID 15362595.
- ↑ "Serotonin Syndrome: Pathophysiology, Clinical Features, Management, and Potential Future Directions". Int J Tryptophan Res 12. 2019. doi:10.1177/1178646919873925. PMID 31523132.
- ↑ "Pharmacotherapy of Patients Taking New Psychoactive Substances: A Systematic Review and Analysis of Case Reports". Front Psychiatry 12. 2021. doi:10.3389/fpsyt.2021.669921. PMID 33967865.
- ↑ "Cyproheptadine for Serotonin Toxicity: an Updated Systematic Review and Grading of Evidence". Current Emergency and Hospital Medicine Reports (Springer Science and Business Media LLC) 8 (4): 151–159. 10 October 2020. doi:10.1007/s40138-020-00222-5. ISSN 2167-4884.
- ↑ "The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity". QJM 96 (9): 635–642. September 2003. doi:10.1093/qjmed/hcg109. PMID 12925718.
- ↑ "Recognition and treatment of serotonin syndrome". Canadian Family Physician 54 (7): 988–992. July 2008. PMID 18625822.
- ↑ "The serotonin syndrome". The New England Journal of Medicine 352 (11): 1112–1120. March 2005. doi:10.1056/NEJMra041867. PMID 15784664.
- ↑ 161.0 161.1 Principles of cardiac toxicology. Boca Raton: CRC Press. 1991. ISBN 978-0-8493-8809-5. https://books.google.com/books?id=AW7M6jBixj4C&pg=PA626. Retrieved 3 February 2010.
- ↑ "Pergolide and Cabergoline But not Lisuride Exhibit Agonist Efficacy at Serotonin 5-HT2B Receptors". http://userpage.fu-berlin.de/~hpertz/Presentation001.pdf.
- ↑ Adverse Drug Reactions Advisory Committee, Australia (2004). "Cardiac valvulopathy with pergolide". Aust Adv Drug React Bull 23 (4). http://www.tga.gov.au/adr/aadrb/aadr0408.htm.
- ↑ "Dopamine agonists and the risk of cardiac-valve regurgitation". The New England Journal of Medicine 356 (1): 29–38. January 2007. doi:10.1056/NEJMoa062222. PMID 17202453.
- ↑ "Valvular heart disease and the use of dopamine agonists for Parkinson's disease". The New England Journal of Medicine 356 (1): 39–46. January 2007. doi:10.1056/NEJMoa054830. PMID 17202454.
- ↑ "Food and Drug Administration Public Health Advisory". 29 March 2007. https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm152695.htm.
- ↑ "Secretory Hormones of Entamoeba histolytica". Ciba Foundation Symposium. Novartis Foundation Symposia. 112. 1985. pp. 139–154. doi:10.1002/9780470720936.ch8. ISBN 978-0-470-72093-6.
- ↑ "Neurohumoral alterations and their role in amoebiasis". Indian Journal of Clinical Biochemistry 20 (2): 142–145. July 2005. doi:10.1007/BF02867414. PMID 23105547.
- ↑ "Effect of exogenous 5-hydroxytryptamine on pathogenicity of Entamoeba histolytica in experimental animals". Indian Journal of Experimental Biology 27 (8): 718–720. August 1989. PMID 2561282.
- ↑ "Latest on Enzymology of Serotonin Biosynthesis in Walnut Seeds". Tryptophan, Serotonin, and Melatonin. Advances in Experimental Medicine and Biology. 467. 1999. pp. 637–644. doi:10.1007/978-1-4615-4709-9_81. ISBN 978-0-306-46204-7.
- ↑ "Antifouling properties of simple indole and purine alkaloids from the Mediterranean gorgonian Paramuricea clavata". Journal of Natural Products 74 (10): 2304–2308. October 2011. doi:10.1021/np200537v. PMID 21939218. Bibcode: 2011JNAtP..74.2304P.
- ↑ "Determination of serotonin and its precursors in chocolate samples by capillary liquid chromatography with mass spectrometry detection". Journal of Chromatography A 1232: 158–165. April 2012. doi:10.1016/j.chroma.2011.11.037. PMID 22186492.
- ↑ "Serotonin, a tryptophan-derived signal conserved in plants and animals, regulates root system architecture probably acting as a natural auxin inhibitor in Arabidopsis thaliana". Plant & Cell Physiology (Oxford University Press (OUP)) 52 (3): 490–508. March 2011. doi:10.1093/pcp/pcr006. PMID 21252298.
- ↑ 174.0 174.1 "The Fusarium crown rot pathogen Fusarium pseudograminearum triggers a suite of transcriptional and metabolic changes in bread wheat (Triticum aestivum L.)". Annals of Botany (Oxford University Press (OUP)) 119 (5): 853–867. March 2017. doi:10.1093/aob/mcw207. PMID 27941094.
- ↑ "The necrotrophic effector SnToxA induces the synthesis of a novel phytoalexin in wheat". The New Phytologist (Wiley) 200 (1): 185–200. October 2013. doi:10.1111/nph.12356. PMID 23782173. Bibcode: 2013NewPh.200..185D.
- ↑ "Differential gene expression and metabolomic analyses of Brachypodium distachyon infected by deoxynivalenol producing and non-producing strains of Fusarium graminearum". BMC Genomics (BioMed Central) 15 (1). July 2014. doi:10.1186/1471-2164-15-629. PMID 25063396.
- ↑ "Hydroxycinnamate Amides: Intriguing Conjugates of Plant Protective Metabolites". Trends in Plant Science (Cell Press) 26 (2): 184–195. February 2021. doi:10.1016/j.tplants.2020.09.011. PMID 33036915. Bibcode: 2021TPS....26..184Z.
- ↑ "Occurrence of serotonin in a hallucinogenic mushroom". Science 128 (3326): 718. September 1958. doi:10.1126/science.128.3326.718. PMID 13580242. Bibcode: 1958Sci...128..718T.
- ↑ "Effects Of 5-HT (Serotonin) On Reproductive Behaviour In Heterodera Schachtii (Nematoda)". Canadian Journal of Zoology 79 (9): 1727. 2001. doi:10.1139/z01-135. Bibcode: 2001CaJZ...79.1727J.
- ↑ "C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway". Neuron 26 (3): 619–631. June 2000. doi:10.1016/S0896-6273(00)81199-X. PMID 10896158.
- ↑ "Serotonin regulates repolarization of the C. elegans pharyngeal muscle". The Journal of Experimental Biology 206 (Pt 2): 223–231. January 2003. doi:10.1242/jeb.00101. PMID 12477893. Bibcode: 2003JExpB.206..223N.
- ↑ "RNAi and functional genomics in plant parasitic nematodes". Annual Review of Phytopathology (Annual Reviews) 47 (1): 207–232. 2009. doi:10.1146/annurev.phyto.112408.132605. PMID 19400649. Bibcode: 2009AnRvP..47..207R. "Octopamine and serotonin regulates the activity of the M3 neurons that direct contraction of the pharynx during C. elegans feeding... Soaking Meloidogyne J2 in dsRNA in the presence of ... resorcinol plus serotonin resulted in uptake of solutions and silencing of genes expressed in the intestine and esophageal glands.".
- ↑ "The effect of social experience on serotonergic modulation of the escape circuit of crayfish". Science 271 (5247): 366–369. January 1996. doi:10.1126/science.271.5247.366. PMID 8553075. Bibcode: 1996Sci...271..366Y.
- ↑ "The toxicogenomic multiverse: convergent recruitment of proteins into animal venoms". Annual Review of Genomics and Human Genetics (Annual Reviews) 10 (1): 483–511. 2009. doi:10.1146/annurev.genom.9.081307.164356. PMID 19640225.
- ↑ "Serotonin, serotonin receptors and their actions in insects". Neurotransmitter 2: 1–14. 2015. doi:10.14800/nt.314.
- ↑ 186.0 186.1 186.2 "Neuropeptides as Regulators of Behavior in Insects". Annual Review of Entomology (Annual Reviews) 62 (1): 35–52. January 2017. doi:10.1146/annurev-ento-031616-035500. PMID 27813667.
- ↑ 187.0 187.1 "Molecular mechanisms of phase change in locusts". Annual Review of Entomology (Annual Reviews) 59 (1): 225–244. 2014-01-07. doi:10.1146/annurev-ento-011613-162019. PMID 24160426. "
p. 231,
The change in the number of several potential neurotransmitters ... such as serotonin... may play an important role in remodeling the CNS during phase change (26, 56, 80).
p. 233,
In the locust S. gregaria, the amount of serotonin in the thoracic ganglia was positively correlated with the extent of gregarious behavior induced by different periods of crowding. A series of pharmacological and behavioral experiments demonstrated that serotonin plays a key role in inducing initial behavioral gregarization (2, 80). However, serotonin is not responsible for maintaining gregarious behavior because its amount in long-term gregarious locusts is less than half that in long-term solitarious locusts (80). In L. migratoria, the injection of serotonin can also slightly initiate gregarious behavior, but serotonin when accompanying crowding treatment induced more solitarious-like behavior than did serotonin injection alone (48). Significant differences in serotonin levels were not found in brain tissues between the two phases of L. migratoria. A recent report by Tanaka & Nishide (97) measured attraction/avoidance behavior in S. gregaria after single and multiple injections of serotonin at different concentrations. Serotonin had only a short-term effect on the level of some locomotor activities and was not involved in the control of gregarious behavior (97). In addition, it is not clear how the neurotransmitter influences this unique behavior, because a binary logistic regression model used in these studies for the behavioral assay focused mostly on only one behavioral parameter representing an overall phase state. Obviously, behavioral phase change might involve alternative regulatory mechanisms in different locust species. Therefore, these studies demonstrate that CNS regulatory mechanisms governing initiation and maintenance of phase change are species specific and involve the interactions between these neurotransmitters.
Given the key roles of aminergic signaling, what are the downstream pathways involved in the establishment of long-term memory? Ott et al. (63) investigated the role of [] protein kinase[] in the phase change in S. gregaria: ... cAMP-dependent protein kinase A (PKA). Through use of pharmacological and RNAi intervention, these authors have demonstrated that PKA... has a critical role in modulating the propensity of locusts to acquire and express gregarious behavior. ... Unfortunately, although a correlation between serotonin and PKA was hypothesized, direct evidence was not provided.". - ↑ 188.0 188.1 "Locust and Grasshopper Management". Annual Review of Entomology (Annual Reviews) 64 (1): 15–34. January 2019. doi:10.1146/annurev-ento-011118-112500. PMID 30256665. "...gregarization is evoked by... tactile stimulation... Tactile stimuli trigger the increase of biogenic amines, particularly serotonin, in the locust nervous system (1, 116); these amines play critical roles in the neurophysiology of locust behavioral phase change.".
- ↑ 189.0 189.1 "Serotonin mediates behavioral gregarization underlying swarm formation in desert locusts". Science 323 (5914): 627–630. January 2009. doi:10.1126/science.1165939. PMID 19179529. Bibcode: 2009Sci...323..627A.
- "Locust swarms 'high' on serotonin". BBC News. 29 January 2009. https://news.bbc.co.uk/2/hi/science/nature/7858996.stm.
- ↑ "Serotonin is critical for rewarded olfactory short-term memory in Drosophila". Journal of Neurogenetics 26 (2): 238–244. June 2012. doi:10.3109/01677063.2012.666298. PMID 22436011.
- ↑ "Chemical codes for the control of behaviour in arthropods". Nature 337 (6202): 33–39. January 1989. doi:10.1038/337033a0. PMID 2562906. Bibcode: 1989Natur.337...33B.
- ↑ "Design and synthesis of novel insecticides based on the serotonergic ligand 1-[(4-aminophenyl)ethyl]-4-[3-(trifluoromethyl)phenyl]piperazine (PAPP)". Journal of Agricultural and Food Chemistry 58 (5): 2624–2629. March 2010. doi:10.1021/jf902640u. PMID 20000410. Bibcode: 2010JAFC...58.2624C.
- ↑ Toxicological Chemistry and Biochemistry (3rd ed.). CRC Press. 2002. p. 393. ISBN 978-1-4200-3212-3.
- ↑ "The nociceptive and anti-nociceptive effects of bee venom injection and therapy: a double-edged sword". Progress in Neurobiology 92 (2): 151–183. October 2010. doi:10.1016/j.pneurobio.2010.06.006. PMID 20558236.
- ↑ "Neurotoxic Animal Poisons and Venoms". Clinical Neurotoxicology. W.B. Saunders. 2009. pp. 463–489. doi:10.1016/B978-032305260-3.50049-6. ISBN 978-0-323-05260-3. http://www.sciencedirect.com/science/article/pii/B9780323052603500496.
- ↑ "Plasticity in Insect Olfaction: To Smell or Not to Smell?". Annual Review of Entomology (Annual Reviews) 61 (1): 317–333. 2016-03-11. doi:10.1146/annurev-ento-010715-023523. PMID 26982441.
- ↑ "Serotonin and neuropeptide F have opposite modulatory effects on fly aggression". Nature Genetics 39 (5): 678–682. May 2007. doi:10.1038/ng2029. PMID 17450142.
- ↑ "The adult Dipteran crop: a unique and overlooked organ". Annual Review of Entomology (Annual Reviews) 58 (1): 205–225. 2013-01-07. doi:10.1146/annurev-ento-120811-153653. PMID 23317042.
- ↑ 200.0 200.1 200.2 200.3 200.4 "The spectrum of behaviors influenced by serotonin". Biological Psychiatry 44 (3): 151–162. August 1998. doi:10.1016/s0006-3223(98)00139-5. PMID 9693387.
- ↑ 201.0 201.1 201.2 "Serotonin Coordinates Responses to Social Stress-What We Can Learn from Fish". Frontiers in Neuroscience 11. 2017-10-25. doi:10.3389/fnins.2017.00595. PMID 29163002.
- ↑ "The expanded biology of serotonin". Annual Review of Medicine 60 (1): 355–366. 2009-02-01. doi:10.1146/annurev.med.60.042307.110802. PMID 19630576.
- ↑ "Daily fluctuation of urine serotonin and cortisol in healthy shelter dogs and influence of intraspecific social exposure". Physiology & Behavior 206: 1–6. July 2019. doi:10.1016/j.physbeh.2019.03.016. PMID 30898540.
- ↑ "Serotonin and Tryptophan Serum Concentrations in Shelter Dogs Showing Different Behavioural Responses to a Potentially Stressful Procedure". Veterinary Sciences 8 (1): 1. December 2020. doi:10.3390/vetsci8010001. PMID 33374183.
- ↑ "Short-term effect of ovariohysterectomy on urine serotonin, cortisol, testosterone and progesterone in bitches". BMC Research Notes 14 (1). July 2021. doi:10.1186/s13104-021-05680-y. PMID 34246304.
- ↑ 206.0 206.1 206.2 "Role of brain serotonin in modulating fish behavior". Current Zoology 62 (3): 317–323. June 2016. doi:10.1093/cz/zow037. PMID 29491919.
- ↑ 207.0 207.1 207.2 207.3 "Serotonergic modulation of social interactions in isolated male mice". Psychopharmacology 97 (2): 154–156. 1989-02-01. doi:10.1007/BF00442239. PMID 2498921.
- ↑ "Serotonin and aggressive motivation in crustaceans: altering the decision to retreat". Proceedings of the National Academy of Sciences of the United States of America 94 (11): 5939–5942. May 1997. doi:10.1073/pnas.94.11.5939. PMID 9159179. Bibcode: 1997PNAS...94.5939H.
- ↑ "The Role of Serotonin (5-HT) in Behavioral Control: Findings from Animal Research and Clinical Implications". The International Journal of Neuropsychopharmacology 18 (10). May 2015. doi:10.1093/ijnp/pyv050. PMID 25991656.
- ↑ 210.0 210.1 210.2 210.3 "Putting it in Context: Linking Auditory Processing with Social Behavior Circuits in the Vertebrate Brain". Integrative and Comparative Biology 57 (4): 865–877. October 2017. doi:10.1093/icb/icx055. PMID 28985384.
- ↑ "Serotonin-deficient mutants and male mating behavior in the nematode Caenorhabditis elegans". The Journal of Neuroscience 13 (12): 5407–5417. December 1993. doi:10.1523/JNEUROSCI.13-12-05407.1993. PMID 8254383.
- ↑ "Mate searching in Caenorhabditis elegans: a genetic model for sex drive in a simple invertebrate". The Journal of Neuroscience 24 (34): 7427–7434. August 2004. doi:10.1523/JNEUROSCI.1746-04.2004. PMID 15329389.
- ↑ 213.0 213.1 "Serotonin receptors antagonistically modulate Caenorhabditis elegans longevity". Aging Cell 6 (4): 483–488. August 2007. doi:10.1111/j.1474-9726.2007.00303.x. PMID 17559503.
- ↑ "A nucleostemin family GTPase, NS3, acts in serotonergic neurons to regulate insulin signaling and control body size". Genes & Development 22 (14): 1877–1893. July 2008. doi:10.1101/gad.1670508. PMID 18628395.
- ↑ "Serotonin and insulin signaling team up to control growth in Drosophila". Genes & Development 22 (14): 1851–1855. July 2008. doi:10.1101/gad.1700708. PMID 18628391.
- ↑ "Effect of exposure to selective serotonin reuptake inhibitors in utero on fetal growth: potential role for the IGF-I and HPA axes". Pediatric Research 65 (2): 236–241. February 2009. doi:10.1203/PDR.0b013e318193594a. PMID 19262294.
- ↑ "Molecular and sensory basis of a food related two-state behavior in C. elegans". PLOS ONE 4 (10). October 2009. doi:10.1371/journal.pone.0007584. PMID 19851507. Bibcode: 2009PLoSO...4.7584B.
- ↑ "Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant". Nature 403 (6769): 560–564. February 2000. doi:10.1038/35000609. PMID 10676966. Bibcode: 2000Natur.403..560S.
- ↑ "Manipulation of serotonin signal suppresses early phase of behavioral aging in Caenorhabditis elegans". Neurobiology of Aging 29 (7): 1093–1100. July 2008. doi:10.1016/j.neurobiolaging.2007.01.013. PMID 17336425.
- ↑ "Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function". Proceedings of the National Academy of Sciences of the United States of America 100 (23): 13525–13530. November 2003. doi:10.1073/pnas.2233056100. PMID 14597720. Bibcode: 2003PNAS..10013525C.
- ↑ "Biotransformation of indole derivatives by mycelial cultures". Zeitschrift für Naturforschung C 63 (1–2): 82–84. 2008. doi:10.1515/znc-2008-1-215. PMID 18386493.
- ↑ "Electrochemical Probes of Microbial Community Behavior". Annual Review of Analytical Chemistry (Annual Reviews) 11 (1): 441–461. June 2018. doi:10.1146/annurev-anchem-061417-125627. PMID 29490192. Bibcode: 2018ARAC...11..441S. "Table 1 The respective potential peaks for various electroactive biomolecules that are produced or consumed by microbes reported in the literaturea ... Serotonin | Indium tin oxide | +0.67 | 66".
- ↑ "THE DARK SIDE OF THE MYCELIUM: Melanins of Phytopathogenic Fungi". Annual Review of Phytopathology (Annual Reviews) 37 (1): 447–471. 1999. doi:10.1146/annurev.phyto.37.1.447. PMID 11701831. Bibcode: 1999AnRvP..37..447H.
- ↑ "Serotonin antagonists". Australian and New Zealand Journal of Medicine 14 (6): 888–895. December 1984. doi:10.1111/j.1445-5994.1984.tb03802.x. PMID 6398056.
- ↑ "Pharmacology of indole-alkylamines". Pharmacological Reviews 6 (4): 425–487. December 1954. doi:10.1016/S0031-6997(25)07372-7. PMID 13236482.
- ↑ "[Vittorio Erspamer (1909-1999)"]. Medicina Nei Secoli 18 (1): 97–113. 2006. PMID 17526278. https://www.medicinaneisecoli.it/index.php/MedSecoli/article/view/491.
- ↑ "Serum vasoconstrictor, serotonin; isolation and characterization". The Journal of Biological Chemistry 176 (3): 1243–1251. December 1948. doi:10.1016/S0021-9258(18)57137-4. PMID 18100415.
- ↑ "Distribution of 5-hydroxytryptamine (serotonin, enteramine) in the wall of the digestive tract". The Journal of Physiology 119 (2–3): 352–362. February 1953. doi:10.1113/jphysiol.1953.sp004850. PMID 13035756.
- ↑ SciFinder – Serotonin Substance Detail. Accessed (4 November 2012).
- ↑ "Serotonin content of some mammalian tissues and urine and a method for its determination". The American Journal of Physiology 175 (1): 157–161. October 1953. doi:10.1152/ajplegacy.1953.175.1.157. PMID 13114371.
- ↑ "Serotonin (5-hydroxytryptamine)". Physiological Reviews (American Physiological Society) 34 (3): 563–588. July 1954. doi:10.1152/physrev.1954.34.3.563. PMID 13185755.
- ↑ 232.0 232.1 "Biochemistry and pharmacology of tryptamines and beta-carbolines. A minireview". J Psychoactive Drugs 16 (4): 347–358. 1984. doi:10.1080/02791072.1984.10472305. PMID 6394730. https://bitnest.netfirms.com/external/10.1080/02791072.1984.10472305. Retrieved 7 April 2025. "Table III: Orally and Parenterally Active Psychotropic Tryptamine Derivatives* [...] Name of Compound: Serotonin [...] Dosage (mg): 100#. Route: Oral. [...] # Cardiovascular and autonomic symptoms; little central activity.".
- ↑ 233.0 233.1 233.2 "5-Methoxy-α-Methyltryptamine (α,O-Dimethylserotonin), A Hallucinogenic Homolog of Serotonin". Biol Psychiatry 15 (2): 349–352. April 1980. PMID 7417623. https://bitnest.netfirms.com/external/Biol.Psychiatry/15.2.349. Retrieved 7 April 2025. "Table I. Human Potency of the Hallucinogenic Tryptamines [...] Name: Serotonin. Dosage: 100c. Route: Oral. Reference: Murphree et al., 1960. [...] c Largely cardiovascular and autonomic distress.".
- ↑ 234.0 234.1 "Comparison of the Effects of Congeners of Lysergic Acid Diethylamide and Tryptophane in Normal Volunteers". Pharmacologist 2 (2): 64. https://bibliography.maps.org/resources/download/9620. "[...] Comparison was made in normal human volunteers of the effects of [...] 5-hydroxytryptamine [...] with [LSD]. The subjects were highly trained to recognize the effects of [LSD] and other hallucinogens. All compounds were given orally. [...] 5-hydroxytryptamine in a dose of 100 mg produced a fall in systolic and a rise in diastolic blood pressures together with a feeling of sedation, abdominal cramping, and muscle aching similar to those of [LSD]. Effects were delayed 6 to 8 hours after dosage. [...]".
- ↑ 235.0 235.1 Erspamer, V. (1961). "Recent Research in the Field of 5-Hydroxytryptamine and Related Indolealkylamines". Fortschritte der Arzneimittelforschung / Progress in Drug Research / Progrès des Recherches Pharmaceutiques. 3. Basel: Birkhäuser Basel. 151–367. doi:10.1007/978-3-0348-7041-2_3. ISBN 978-3-0348-7043-6. "13.9 Indolealkylamines and the CNS in human beings 13.91 Central effects of 5-HT [...]"
- ↑ 236.0 236.1 "The effects of certain drugs on cerebral synapses". Ann N Y Acad Sci 66 (3): 496–507. March 1957. doi:10.1111/j.1749-6632.1957.tb40745.x. PMID 13425238. Bibcode: 1957NYASA..66..496M. "Unfortunately, except for the intraventricular injections described by Sherwood,7 there have been, thus far, no documented reports of serotonin-induced mental disturbance* in man that are clearly separable from the natural anxiety initiated by the profound peripheral effects such as circulatory disturbance, other autonomic effects, and emesis. [...] 7. SHERWOOD, S, L. 1955. The responses of psychotic patients to intraventricular injections. Proc. Roy. Soc. Med. 48: 855.".
- ↑ "Recent experiments with injections of drugs into the ventricular system of the brain: The Response of Psychotic Patients to Intraventricular Injections". Proc R Soc Med 48 (10): 853–864. October 1955. doi:10.1177/003591575504801031. PMID 13266838. "Serotonin. [...] 4 patients received this drug in doses of 15 to 75 μg. [...]".
- ↑ 238.0 238.1 238.2 238.3 Alexander T. Shulgin; Ann Shulgin (1997). TiHKAL: The Continuation (1st ed.). Berkeley, CA: Transform Press. ISBN 978-0-9630096-9-2. OCLC 38503252. https://books.google.com/books?id=jl_ik66IumUC. Retrieved 30 January 2025. "Two obstacles effectively prohibit this availability. Serotonin has a free hydroxy group (the 5-hydroxy which is the H of 5-HT). This is a big polar water-loving pimple which denies it any passage across the brain's defensive Maginot Line, the blood-brain barrier. And there is the second problem. There is a exposed amino group, the amine of T of 5-HT, the tryptamine, which is immediately removed by the body's monoamine oxidase enzyme. In short, it is blocked from entry into the brain because it is both too polar and too metabolically fragile."
- ↑ 239.0 239.1 "The hallucinogenic world of tryptamines: an updated review". Arch Toxicol 89 (8): 1151–1173. August 2015. doi:10.1007/s00204-015-1513-x. PMID 25877327. Bibcode: 2015ArTox..89.1151A. https://psilosybiini.info/paperit/The%20hallucinogenic%20world%20of%20tryptamines,%20an%20updated%20review%20(Ara%FAjo%20et%20al.,%202015).pdf.
- ↑ "Serotonergic Psychedelics: A Comparative Review of Efficacy, Safety, Pharmacokinetics, and Binding Profile". Biol Psychiatry Cogn Neurosci Neuroimaging 9 (5): 472–489. May 2024. doi:10.1016/j.bpsc.2024.01.007. PMID 38301886.
Further reading
- "Tryptophan hydroxylase-2 gene variation influences personality traits and disorders related to emotional dysregulation". The International Journal of Neuropsychopharmacology 10 (3): 309–320. June 2007. doi:10.1017/S1461145706007437. PMID 17176492.
External links
- 5-Hydroxytryptamine MS Spectrum
- Serotonin bound to proteins in the PDB
- PsychoTropicalResearch Extensive reviews on serotonergic drugs and Serotonin Syndrome.
- Molecule of the Month: Serotonin at University of Bristol
- 60-Second Psych: No Fair! My Serotonin Level Is Low , Scientific American
- Serotonin Test Interpretation on ClinLab Navigator .
Template:Chemical classes of psychoactive drugs
 |
 KSF
KSF