Silvestrol
Topic: Chemistry
 From HandWiki - Reading time: 2 min
From HandWiki - Reading time: 2 min
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C34H38O13 |
| Molar mass | 654.7 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
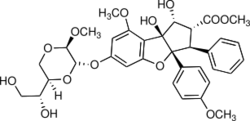
Silvestrol is a natural product from the flavagline family, with a cyclopenta[b] benzofuran core structure and an unusual dioxane ether side chain, which is found in the bark of trees from the genus Aglaia, especially Aglaia silvestris and Aglaia foveolata.[1]
Bioactivity
It acts as a potent and selective inhibitor of the RNA helicase enzyme eIF4A, and has both broad-spectrum antiviral activity against diseases such as Ebola and coronaviruses, [2][3][4][5][6] and anti-cancer properties,[7][8] which makes it of considerable interest in medical research. However, as it cannot be extracted from tree bark in commercial amounts and is prohibitively complex to produce synthetically,[9] practical applications have focused more on structurally simplified analogues such as CR-31-B.[10]
See also
References
- ↑ "Rocaglamide, silvestrol and structurally related bioactive compounds from Aglaia species". Natural Product Reports 31 (7): 924–39. July 2014. doi:10.1039/c4np00006d. PMID 24788392.
- ↑ "The natural compound silvestrol is a potent inhibitor of Ebola virus replication". Antiviral Research 137: 76–81. January 2017. doi:10.1016/j.antiviral.2016.11.011. PMID 27864075.
- ↑ "Inhibition of Zika Virus Replication by Silvestrol". Viruses 10 (4): 149. March 2018. doi:10.3390/v10040149. PMID 29584632.
- ↑ "Broad-spectrum antiviral activity of the eIF4A inhibitor silvestrol against corona- and picornaviruses". Antiviral Research 150: 123–129. February 2018. doi:10.1016/j.antiviral.2017.12.010. PMID 29258862.
- ↑ "Silvestrol Inhibits Chikungunya Virus Replication". Viruses 10 (11): 592. October 2018. doi:10.3390/v10110592. PMID 30380742.
- ↑ "Recent discovery and development of inhibitors targeting coronaviruses". Drug Discovery Today 25 (4): 668–688. April 2020. doi:10.1016/j.drudis.2020.01.015. PMID 32006468.
- ↑ "Therapeutic potential of the translation inhibitor silvestrol in hepatocellular cancer". PLOS ONE 8 (9): e76136. 2013. doi:10.1371/journal.pone.0076136. PMID 24086701. Bibcode: 2013PLoSO...876136K.
- ↑ "Targeting the eIF4F translation initiation complex: a critical nexus for cancer development". Cancer Research 75 (2): 250–63. January 2015. doi:10.1158/0008-5472.CAN-14-2789. PMID 25593033.
- ↑ "Total synthesis of (-)-episilvestrol and (-)-silvestrol". Angewandte Chemie 46 (41): 7835–8. 2007. doi:10.1002/anie.200702700. PMID 17823902.
- ↑ "Comparison of broad-spectrum antiviral activities of the synthetic rocaglate CR-31-B (-) and the eIF4A-inhibitor Silvestrol". Antiviral Research 175: 104706. January 2020. doi:10.1016/j.antiviral.2020.104706. PMID 31931103.
 |
 KSF
KSF