Stabilizer
Topic: Chemistry
 From HandWiki - Reading time: 2 min
From HandWiki - Reading time: 2 min
In industrial chemistry, a stabilizer or stabiliser is a chemical that is used to prevent degradation.[1]
- Representative stabilizers
-
Tris(2,4-di-tert-butylphenyl)phosphite is a widely used stabilizer in polymers.
-
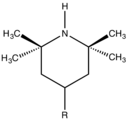
Partial structure of typical hindered amine light stabilizers, widely used to protect automotive paints from degradation by UV-light.
-
Salpn is a typical metal deactivator used as a fuel additive to suppress oxidation processes that lead to gums and solids. Metal deactivators like salpn form stable complexes with the metals, suppressing their catalytic activity.[2]
Overview
Heat and light stabilizers are added to plastics because they ensure safe processing and protect products against aging and weathering. The trend is towards fluid systems, pellets, and increased use of masterbatches. There are monofunctional, bifunctional, and polyfunctional stabilizers. In economic terms the most important product groups on the market for stabilizers are compounds based on calcium (calcium-zinc and organo-calcium), lead, and tin stabilizers as well as liquid and light stabilizers (HALS, benzophenone, benzotriazole). Cadmium-based stabilizers largely vanished in the last years due to health and environmental concerns.[3]
Polymers
Some kinds of stabilizers are:
- antioxidants these prevent autoxidation of materials and come in 3 primary forms.
- Oxygen scavengers (primarily phosphite esters such as tris(2,4-di-tert-butylphenyl)phosphite) are commonly used during the initial processing of the plastic.
- Persistent radical scavengers prevent or slow the photo-oxidation of polymers. Traditionally these are alkylated phenols such as butylated hydroxytoluene but now also include hindered amine light stabilizers (HALS)
- Antiozonants prevents or retards the degradation of polymers caused by ozone (ozone cracking)
- sequestrants, forming chelate complexes and inactivating traces of metal ions that would otherwise act as catalysts
- ultraviolet stabilizers are used to protect polymers from effects of ultraviolet radiation and come to 2 main types.
- UV absorbers which essentially act the same way as sunscreens
- Quenchers, which dissipate the radiation energy as heat instead of letting it break chemical bonds; often organic nickel salts, e.g. nickel phenolates
Paints
- emulsifiers and surfactants, for stabilization of emulsions[citation needed]
Food
In foods, stabilizers prevent spoilage. Classes of food stabilizers include emulsifiers, thickeners and gelling agents, foam stabilizers, humectants, anticaking agents, and coating agents.[4]
See also
- Corrosion inhibitor
- Stabilizers for polymers
References
- ↑ Rainer Wolf; Bansi Lal Kaul (2000). "Plastics, Additives". Ullmann's Encyclopedia Of Industrial Chemistry. doi:10.1002/14356007.a20_459. ISBN 3527306730.
- ↑ Dabelstein, W.; Reglitzky A.; Schutze A.; Reders, K.. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH.
- ↑ "Market Study: Stabilizers (UC-7405)". March 2014. http://www.ceresana.com/en/market-studies/additives/stabilizers/.
- ↑ Erich Lück, Gert-Wolfhard von Rymon Lipinski "Foods, 3. Food Additives" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a11_561
 KSF
KSF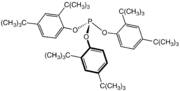
![Salpn is a typical metal deactivator used as a fuel additive to suppress oxidation processes that lead to gums and solids. Metal deactivators like salpn form stable complexes with the metals, suppressing their catalytic activity.[2]](https://handwiki.org/wiki/images/thumb/9/9c/Salpn.png/180px-Salpn.png)
