Synergistic catalysis
Topic: Chemistry
 From HandWiki - Reading time: 4 min
From HandWiki - Reading time: 4 min
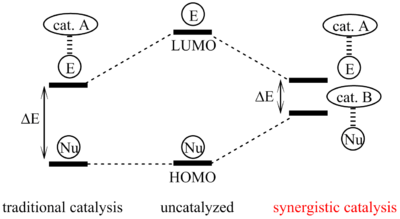
Synergistic catalysis is a specialized approach to catalysis whereby at least two different catalysts act on two different substrates simultaneously to allow reaction between the two activated materials. While a catalyst works to lower the energy of reaction overall, a reaction using synergistic catalysts work together to increase the energy level of HOMO of one of the molecules and lower the LUMO of another.[1] While this concept has come to be important in developing synthetic pathways, this strategy is commonly found in biological systems as well.
Background
Synergistic catalysts have been used for a variety of reactions, especially when both substrates require some kind of significant activation either with stoichiometric amounts of an activator or through a separate reaction beforehand. Synergistic catalysts differ from other multi-catalyst systems by the nature that one catalyst activates one substrate while the other activates a different substrate. There are other types of multi-catalyst systems such as double activation catalysts where two catalysts are required to activate one substrate or cascade catalysts where one catalyst first transforms a substrate which then is activated by a second catalyst to react.[2][3][4]
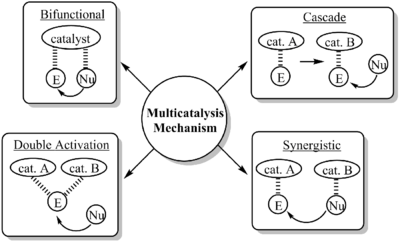
While this field does show particular promise in affording molecules that could not be synthesized under normal synthetic strategies, there are a few issues that need to be addressed. One such issue is self quenching of the catalysts with each other. An example is if one of the catalysts is a Lewis acid and the other is a Lewis base, there is the possibility for formation of a Lewis acid base complex but this can be overcome by carefully choosing the pair.[5]
Examples
In Biology
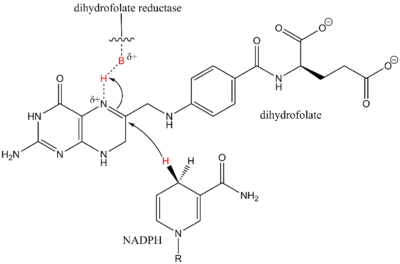
Synergistic catalysts are very common in biological systems.[6] The reactions occur by a molecule binding to a protein as a substrate and becoming active and being reacted with a coenzyme such as NADPH which is essentially an activated hydride. A specific example of this is shown by the synthesis of tetrahydrofolate via the enzyme dihydrofolate reductase. Dihydrofolate reductase catalytically activates dihydrofolate by protonating the imine, while NADPH, essentially a hydride source activated by the cofactor NADP+, can then come in and add a hydride across the imine to afford the product.[7]
Dual Transition Metals Catalysis
Through the combination of two transition metal catalysts, synergistic catalysis has been reported to accelerate many chemical transformations, and even to induce high enantioselectivity, which could not be realized by the use individual catalysts. Sawamura et al. reported an early example of enantioselective allylic alkylation of nitriles catalyzed by a mixture of rhodium and palladium complexes.[8] The palladium catalyst with chiral ligands alone gave a high yield, but no enantioselectivity was observed. The reaction did not proceed at all using the rhodium catalyst alone. Using both together, however, gave both a high yield and enantioselectivity for the transformation.
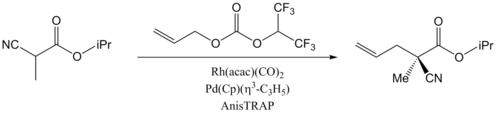
They used trans-chelating chiral phosphine ligands (AnisTRAP) to generate chiral transition metal complexes. In their proposed mechanism schemes, an enolate is formed from an α-cyano ester and coordinates to the rhodium catalyst, while decarboxylative and oxidative addition of allyl carbonate to the palladium catalyst forms the π-allylpalladium (II) complex. Subsequently, the enolate attacks the π-allylpalladium (II) complex enantioselectively to afford the optically active product.
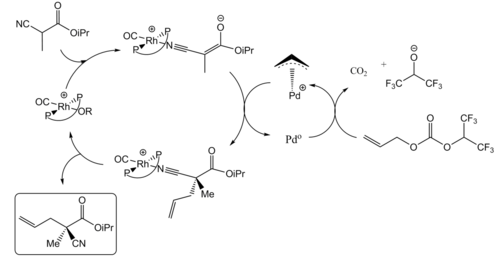
Enantio- and Diastereoselective Catalysis
Besides using two transition metal catalysts, synergistic catalysis can also be carried out by utilizing one transition metal catalyst in combination with an organocatalyst. Here the synergistic α-allylation of aldehydes was accomplished by utilizing a transition metal complex in combination with a chiral amine catalyst.[9][10] In 2013, Carreira and co-workers reported a highly enantio- and diastereoselective α-allylation of branched aldehydes.[11] They used chiral primary amines and iridium catalysts complexed with chiral ligands to afford the product with two newly formed stereocenters at the α and β position.
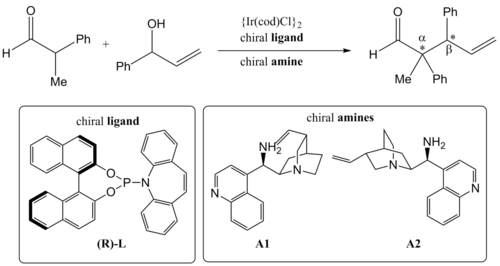
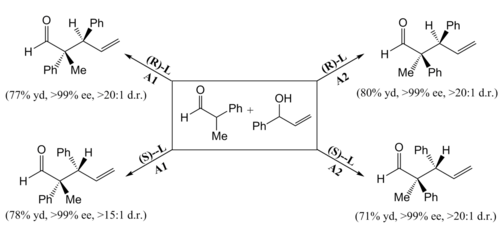
By matching the two chiral amines and enantiomers of the chiral ligands, they were able to access all four possible stereoisomers of the product with good yields. More importantly, their catalytic system exhibits simultaneous and almost absolute control over the stereochemical configurations of both stereocenters.
References
- ↑ Allen, Anna E.; MacMillan, David W. C. (1 January 2012). "Synergistic catalysis: A powerful synthetic strategy for new reaction development". Chemical Science 2012 (3): 633. doi:10.1039/c2sc00907b. PMID 22518271.
- ↑ Xu, H.; Zuend, S. J.; Woll, M. G.; Tao, Y.; Jacobsen, E. N. (18 February 2010). "Asymmetric Cooperative Catalysis of Strong Bronsted Acid-Promoted Reactions Using Chiral Ureas". Science 327 (5968): 986–990. doi:10.1126/science.1182826. PMID 20167783. Bibcode: 2010Sci...327..986X.
- ↑ Belot, Sebastien; Vogt, Kim A.; Besnard, Celine; Krause, Norbert; Alexakis, Alexandre (9 November 2009). "Enantioselective One-Pot Organocatalytic Michael Addition/Gold-Catalyzed Tandem Acetalization/Cyclization". Angewandte Chemie International Edition 48 (47): 8923–8926. doi:10.1002/anie.200903905. PMID 19847839.
- ↑ Swiegers, Gerhard F. (2008). Mechanical catalysis : methods of enzymatic, homogeneous, and heterogeneous catalysis ([Online Resource] ed.). Hoboken, N.J.: John Wiley. pp. 181–207. ISBN 9780470262023. https://archive.org/details/mechanicalcataly00swie.
- ↑ Pearson, Ralph G. (1 November 1963). "Hard and Soft Acids and Bases". Journal of the American Chemical Society 85 (22): 3533–3539. doi:10.1021/ja00905a001.
- ↑ Sträter, Norbert; Lipscomb, William N.; Klabunde, Thomas; Krebs, Bernt (1 October 1996). "Two-Metal Ion Catalysis in Enzymatic Acyl- and Phosphoryl-Transfer Reactions". Angewandte Chemie International Edition in English 35 (18): 2024–2055. doi:10.1002/anie.199620241.
- ↑ Brown, Katherine A.; Kraut, Joseph (1 January 1992). "Exploring the molecular mechanism of dihydrofolate reductase". Faraday Discussions 93: 217. doi:10.1039/FD9929300217. Bibcode: 1992FaDi...93..217B.
- ↑ Sawamura, Masaya; Sudoh, Masaki; Ito, Yoshihiko (1 January 1996). "An Enantioselective Two-Component Catalyst System: Rh−Pd-Catalyzed Allylic Alkylation of Activated Nitriles". Journal of the American Chemical Society 118 (13): 3309–3310. doi:10.1021/ja954223e.
- ↑ Ibrahem, I.; Córdova, A. (6 March 2006). "Direct Catalytic Intermolecular -Allylic Alkylation of Aldehydes by Combination of Transition-Metal and Organocatalysis". Angew. Chem. Int. Ed. 45: 1952. doi:10.1002/anie.200504021.
- ↑ Afewerki, S.; Ibrahem, I.; Rydfjord, J.; Breistein, P.; Córdova, A. (5 March 2012). "Direct Regiospecific and Highly Enantioselective Intermolecular –Allylic Alkylation of Aldehydes by a Combination of Transition-Metal and Chiral Amine Catalysts". Chem. Eur. J. 18: 2972. doi:10.1002/chem.201103366.
- ↑ Krautwald, S.; Sarlah, D.; Schafroth, M. A.; Carreira, E. M. (30 May 2013). "Enantio- and Diastereodivergent Dual Catalysis: -Allylation of Branched Aldehydes". Science 340 (6136): 1065–1068. doi:10.1126/science.1237068. PMID 23723229. Bibcode: 2013Sci...340.1065K.
 |
 KSF
KSF