Taurine
Topic: Chemistry
 From HandWiki - Reading time: 11 min
From HandWiki - Reading time: 11 min
Taurine (/ˈtɔːriːn/ (![]() listen);[1] IUPAC: 2-aminoethanesulfonic acid[2]) is a naturally occurring organic compound with the chemical formula C
listen);[1] IUPAC: 2-aminoethanesulfonic acid[2]) is a naturally occurring organic compound with the chemical formula C
2H
7NO
3S, and is a non-proteinogenic amino sulfonic acid widely distributed in mammalian tissues and organs.[2][3] Structurally, by containing a sulfonic acid group instead of a carboxylic acid group, it is not involved in protein synthesis but is still usually referred to as an amino acid.[2][4][5][6] As non-proteinogenic amino sulfonic acid, it is not encoded by the genetic code and is distinguished from the protein-building α-amino acids.[7]
Taurine is a major constituent of bile and can be found in the large intestine, and is named after Latin taurus, meaning bull or ox, as it was first isolated from ox bile in 1827 by German scientists Friedrich Tiedemann and Leopold Gmelin.
Although taurine is abundant in human organs, it is not an essential human dietary nutrient and is not included among nutrients with a recommended intake level.[8] Among the diverse pathways by which natural taurine can be biosynthesized, its human pathways (primarily in the human liver) are from cysteine and/or methionine.[9][10]
Taurine is commonly sold as a dietary supplement, but there is no good clinical evidence that taurine supplements provide any benefit to human health.[11] Taurine is used as a food additive to meet essential dietary intake levels for cats,[12] and supplemental dietary support for dogs and poultry.[13]
Discovery and name
Taurine, named after Latin taurus, meaning bull or ox,[14] was first isolated from ox bile in 1827 by German scientists Friedrich Tiedemann and Leopold Gmelin.[15][16] Another German scientist Von H. Demarcay first used its common chemical name—Taurine—in 1838, derived from the Latin taurus (cognate to Ancient Greek ταῦρος, taûros) meaning bull or ox.[17][14][16]It was subsequently identified in human bile in 1846 by Edmund Ronalds.[18]
In nature
Taurine is widely distributed in nature, particularly in animal tissues.[3] Moreover, it is abundant in nature, including in animal organs,[19][better source needed] and further, as substrates ref name="Ripps_2012">"Taurine: A "Very Essential" Amino Acid" (review). Molecular Vision 18: 2673–2686. 12 November 2012. PMID 23170060.</ref> Taurine concentrations in human cells may derive from at least three processes:
- biosynthesis from the sulfur amino acids (e.g., cysteine);
- active uptake by a possible taurine transporter; and
- the extent of its release from cells by a "volume-sensitive leak pathway".[9]
It is not an essential human dietary nutrient, resulting in the absence of taurine from compounds having a Reference Daily Intake.[8] Its role in human physiology is unknown.
Taurine is a major constituent of bile, and can be found in the large intestine. Its concentrations in land plants are low or undetectable, but up to a substantial wet weight has been found in algae.[20][21]
Chemical and biochemical features
Taurine exists as a zwitterion H
3N+
CH
2CH
2SO−
3, as verified by X-ray crystallography.[22] The sulfonic acid has a low pKa[23] ensuring that it is fully ionized to the sulfonate at the pHs found in the intestinal tract.
Biosynthesis
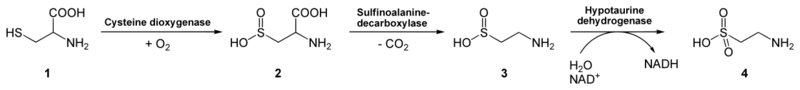
Among the diverse pathways by which natural taurine can be biosynthesized, its pathways in the human liver are from cysteine and/or methionine.[9][10] With regard to the route from cysteine: mammalian taurine synthesis occurs in the liver via the cysteine sulfinic acid pathway. In this pathway, cysteine is first oxidized to its sulfinic acid, catalyzed by the enzyme cysteine dioxygenase. Cysteine sulfinic acid, in turn, is decarboxylated by sulfinoalanine decarboxylase to form hypotaurine. Hypotaurine is enzymatically oxidized to yield taurine by hypotaurine dehydrogenase.[24]
Taurine is also produced by the transsulfuration pathway, which converts homocysteine into cystathionine. The cystathionine is then converted to hypotaurine by the sequential action of three enzymes: cystathionine gamma-lyase, cysteine dioxygenase, and cysteine sulfinic acid decarboxylase. Hypotaurine is then oxidized to taurine as described above.[25]
A pathway for taurine biosynthesis from serine and sulfate is reported in microalgae,[21] developing chicken embryos,[26] and chick liver.[27] Serine dehydratase converts serine to 2-aminoacrylate, which is converted to cysteic acid by 3′-phosphoadenylyl sulfate:2-aminoacrylate C-sulfotransferase. Cysteic acid is converted to taurine by cysteine sulfinic acid decarboxylase.
Chemical synthesis
Synthetic taurine is obtained by the ammonolysis of isethionic acid (2-hydroxyethanesulfonic acid), which in turn is obtained from the reaction of ethylene oxide with aqueous sodium bisulfite. A direct approach involves the reaction of aziridine with sulfurous acid.[28]
In 1993, about 5000–6000 t of taurine were produced for commercial purposes: 50% for pet food and 50% in pharmaceutical applications.[29]
In the laboratory, taurine can be produced by alkylation of ammonia with bromoethanesulfonate salts.[30][needs update?]
In food
Taurine occurs naturally in fish and meat.[11][31][16] The mean daily intake from omnivore diets was determined to be around Lua error: not enough memory. (range Lua error: Internal error: The interpreter exited with status 1.),[32] and to be low or negligible from a vegan diet.[11] Typical taurine consumption in the American diet is about Lua error: Internal error: The interpreter exited with status 1. per day.[11]
Taurine is partially destroyed by heat in processes such as baking and boiling. This is a concern for cat food, as cats have a dietary requirement for taurine and can easily become deficient. Either raw feeding or supplementing taurine can satisfy this requirement.[33][34]
Both lysine and taurine can mask the metallic flavor of potassium chloride, a salt substitute.[35]
Breast milk
Taurine is present in breast milk, and has been added to many infant formulas as a measure of prudence since the early 1980s. However, this practice has never been rigorously studied, and as such it has yet to be proven to be necessary, or even beneficial.[36]
Energy drinks and dietary supplements
Taurine is an ingredient in some energy drinks in amounts of Lua error: Internal error: The interpreter exited with status 1. per serving.[11][37]
Research
Taurine is not regarded as an essential human dietary nutrient and has not been assigned recommended intake levels.[8] High-quality clinical studies to determine possible effects of taurine in the body or following dietary supplementation are absent from the literature.[11] Preliminary human studies on the possible effects of taurine supplementation have been inadequate due to low subject numbers, inconsistent designs, and variable doses.[11]
Safety and toxicity
According to the European Food Safety Authority, taurine is "considered to be a skin and eye irritant and skin sensitiser, and to be hazardous if inhaled"; it may be safe to consume up to 6 grams of taurine per day.[13] Other sources indicate that taurine is safe for supplemental intake in normal healthy adults at up to 3 grams per day.[11][38]
A 2008 review found no documented reports of negative or positive health effects associated with the amount of taurine used in energy drinks, concluding, "The amounts of guarana, taurine, and ginseng found in popular energy drinks are far below the amounts expected to deliver either therapeutic benefits or adverse events".[39]
Animal dietary requirement
Cats
Cats lack the enzyme sulfinoalanine decarboxylase to produce taurine and must therefore acquire it from their diet.[12] A taurine deficiency in cats can lead to retinal degeneration and eventually blindness ‒ a condition known as central retinal degeneration[40][41] as well as hair loss and tooth decay. Other effects of a diet lacking in this essential amino acid are dilated cardiomyopathy,[42] and reproductive failure in female cats.[12][43]
Decreased plasma taurine concentration has been demonstrated to be associated with feline dilated cardiomyopathy. Unlike CRD, the condition is reversible with supplementation.[44]
Taurine is now a requirement of the Association of American Feed Control Officials (AAFCO) and any dry or wet food product labeled approved by the AAFCO should have a minimum of 0.1% taurine in dry food and 0.2% in wet food.[45] Studies suggest the amino acid should be supplied at Lua error: Internal error: The interpreter exited with status 1. of bodyweight per day for domestic cats.[46]
Other mammals
A number of other mammals also have a requirement for taurine. While the majority of dogs can synthesize taurine, case reports have described a singular American cocker spaniel, 19 Newfoundland dogs, and a family of golden retrievers suffering from taurine deficiency treatable with supplementation. Foxes on fur farms also appear to require dietary taurine. The rhesus, cebus and cynomolgus monkeys each require taurine at least in infancy. The giant anteater also requires taurine.[47]
Birds
Taurine appears to be essential for the development of passerine birds. Many passerines seek out taurine-rich spiders to feed their young, particularly just after hatching. Researchers compared the behaviours and development of birds fed a taurine-supplemented diet to a control diet and found the juveniles fed taurine-rich diets as neonates were much larger risk takers and more adept at spatial learning tasks. Under natural conditions, each blue tit nestling receive Lua error: Internal error: The interpreter exited with status 1. of taurine per day from parents.[48]
Taurine can be synthesized by chickens. Supplementation has no effect on chickens raised under adequate lab conditions, but seems to help with growth under stresses such as heat and dense housing.[49]
Fish
Species of fish, mostly carnivorous ones, show reduced growth and survival when the fish-based feed in their food is replaced with soy meal or feather meal. Taurine has been identified as the factor responsible for this phenomenon; supplementation of taurine to plant-based fish feed reverses these effects. Future aquaculture is expected to use more of these more environmentally-friendly protein sources, so supplementation would become more important.[50]
The need of taurine in fish is conditional, differing by species and growth stage. The olive flounder, for example, has lower capacity to synthesize taurine compared to the rainbow trout. Juvenile fish are less efficient at taurine biosyntheis due to reduced cysteine sulfinate decarboxylase levels.[51]
Derivatives
- Taurine is used in the preparation of the anthelmintic drug, Totabin
- Taurolidine
- Taurocholic acid and tauroselcholic acid
- Tauromustine
- 5-Taurinomethyluridine and 5-taurinomethyl-2-thiouridine are modified uridines in (human) mitochondrial tRNA.[52]
- Tauryl is the functional group attaching at the sulfur, 2-aminoethylsulfonyl.[53]
- Taurino is the functional group attaching at the nitrogen, 2-sulfoethylamino.
- Thiotaurine
- Peroxytaurine which is a degradation product by both superoxide and heat degradation.
See also
- Homotaurine (tramiprosate), precursor to acamprosate
- Taurates, a group of surfactants
References
- ↑ "Oxford Learner's Dictionaries -- Taurine". Oxford University Press. https://www.oxfordlearnersdictionaries.com/definition/english/taurine.
- ↑ 2.0 2.1 2.2 "Taurine". https://pubchem.ncbi.nlm.nih.gov/compound/1123.
- ↑ 3.0 3.1 "Taurine: new implications for an old amino acid". FEMS Microbiology Letters 226 (2): 195–202. September 2003. doi:10.1016/S0378-1097(03)00611-6. PMID 14553911. https://academic.oup.com/femsle/article-abstract/226/2/195/577328?redirectedFrom=fulltex.
- ↑ Lehninger, Albert L.; Nelson, David L.; Cox, Michael M. (2013). Lehninger Principles of Biochemistry (6th ed.). New York: W.H. Freeman. p. 730. ISBN 978-1-4292-3414-6. https://archive.org/details/lehningerprincip0000lehn/page/730/mode/2up?q=Taurine. Retrieved July 7, 2025.
- ↑ Voet, Donald; Voet, Judith G.; Pratt, Charlotte W. (2013). Fundamentals of Biochemistry: Life at the Molecular Level (4th ed.). John Wiley & Sons. p. 86. ISBN 978-1-118-12918-0. https://archive.org/details/FundamentalsBiochemistry4e_201802/page/n123/mode/2up?q=Taurine. Retrieved July 7, 2025.
- ↑ Hendler, Sheldon Saul (1990). The Doctors' Vitamin and Mineral Encyclopedia. Simon & Schuster. p. 208. ISBN 978-0671667849. https://archive.org/details/the-doctors-vitamin-and-mineral-encyclopedia/page/208/mode/2up?q=taurine. Retrieved July 7, 2025.
- ↑ Nelson, David L.; Cox, Michael M. (2021). Lehninger Principles of Biochemistry (8th ed.). New York: W.H. Freeman. p. 866. ISBN 978-1-319-22800-2.
- ↑ 8.0 8.1 8.2 "Daily Value on the New Nutrition and Supplement Facts Labels". US Food and Drug Administration. 25 February 2022. https://www.fda.gov/food/new-nutrition-facts-label/daily-value-new-nutrition-and-supplement-facts-labels#referenceguide.
- ↑ 9.0 9.1 9.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedRipps_2012 - ↑ 10.0 10.1 "Taurine". PubChem, US National Library of Medicine. 25 May 2024. https://pubchem.ncbi.nlm.nih.gov/compound/Taurine. Retrieved 31 May 2024.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 11.7 "Taurine". Drugs.com. 15 May 2023. https://www.drugs.com/npp/taurine.html.
- ↑ 12.0 12.1 12.2 "Taurine: an essential nutrient for the cat". The Journal of Nutrition 108 (5): 773–778. May 1978. doi:10.1093/jn/108.5.773. PMID 641594. https://www.researchgate.net/publication/22499901.
- ↑ 13.0 13.1 EFSA Panel on Additives and Products or Substances used in Animal Feed (2012). "Scientific Opinion on the safety and efficacy of taurine as a feed additive for all animal species". EFSA Journal 10 (6): 2736. doi:10.2903/j.efsa.2012.2736.
- ↑ 14.0 14.1 Ripps, Harris; Shen, Wen (2012). "Review: taurine: a "very essential" amino acid". Molecular Vision 18: 2673–2686. ISSN 1090-0535. PMID 23170060.
- ↑ "Einige neue Bestandtheile der Galle des Ochsen". Annalen der Physik 85 (2): 326–337. 1827. doi:10.1002/andp.18270850214. Bibcode: 1827AnP....85..326T. https://zenodo.org/record/1423514.
- ↑ 16.0 16.1 16.2 "Physiological actions of taurine". Physiological Reviews 72 (1): 101–163. January 1992. doi:10.1152/physrev.1992.72.1.101. PMID 1731369. https://journals.physiology.org/doi/epdf/10.1152/physrev.1992.72.1.101.
- ↑ Demarcay, H. (1838). "Ueber die natur der Galle". Journal für Praktische Chemie 15 (1): 193-212. doi:10.1002/prac.18380150118. https://ia800805.us.archive.org/view_archive.php?archive=/13/items/crossref-pre-1909-scholarly-works/10.1002%252Fprac.18380140149.zip&file=10.1002%252Fprac.18380150118.pdf. Retrieved 7 July 2025.
- ↑ "Bringing Together Academic and Industrial Chemistry: Edmund Ronalds' Contribution". Substantia 3 (1): 139–152. 2019. https://riviste.fupress.net/index.php/subs/article/view/211. Retrieved 7 April 2025.
- ↑ "Regulation of the Cellular Content of the Organic Osmolyte Taurine in Mammalian Cells". Neurochemical Research 29 (1): 27–63. 2004. doi:10.1023/b:nere.0000010433.08577.96. PMID 14992263.
- ↑ "Occurrence of Taurine in Plants". Agricultural and Biological Chemistry 50 (7): 1887–1888. 1986. doi:10.1271/bbb1961.50.1887. https://www.jstage.jst.go.jp/article/bbb1961/50/7/50_7_1887/_article.
- ↑ 21.0 21.1 "Amino acid content of selected plant, algae and insect species: a search for alternative protein sources for use in pet foods" (in en). Journal of Nutritional Science 3. 2014. doi:10.1017/jns.2014.33. ISSN 2048-6790. PMID 26101608.
- ↑ "Taurine". Acta Crystallographica Section C 56 (1): e23–e24. 2000. doi:10.1107/S0108270199016029. Bibcode: 2000AcCrC..56E..23G.
- ↑ "13C nuclear magnetic resonance study of the complexation of calcium by taurine". Journal of Inorganic Biochemistry 13 (2): 137–150. October 1980. doi:10.1016/S0162-0134(00)80117-8. PMID 7431022.
- ↑ "Oxidation of hypotaurine in rat liver". Biochimica et Biophysica Acta 63: 210–212. September 1962. doi:10.1016/0006-3002(62)90357-8. PMID 13979247.
- ↑ "Review: taurine: a "very essential" amino acid". Molecular Vision 18: 2673–2686. 2012. PMID 23170060.
- ↑ "The Utilization of Sulfate Sulfur for the Synthesis of Taurine in the Developing Chick Embryo". The Journal of Biological Chemistry 212 (1): 469–475. 1955. doi:10.1016/s0021-9258(18)71134-4. ISSN 0021-9258. PMID 13233249.
- ↑ "The Synthesis of Taurine from Sulfate III. Further Evidence for the Enzymatic Pathway in Chick Liver" (in en). Proceedings of the Society for Experimental Biology and Medicine 139 (3): 755–761. 1972-03-01. doi:10.3181/00379727-139-36232. ISSN 1535-3702. PMID 5023763. http://ebm.sagepub.com/lookup/doi/10.3181/00379727-139-36232.
- ↑ "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. 2000. doi:10.1002/14356007.a25_503.
- ↑ Tully, Paul S. (2000). "Sulfonic Acids". Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. doi:10.1002/0471238961.1921120620211212.a01. ISBN 978-0-471-23896-6.
- ↑ "Taurine". Organic Syntheses 18: 77. 1938. doi:10.15227/orgsyn.018.0077.
- ↑ "The sulfur-containing amino acids: an overview". The Journal of Nutrition 136 (6 Suppl): 1636S–1640S. June 2006. doi:10.1093/jn/136.6.1636S. PMID 16702333.
- ↑ "Opinion on Caffeine, Taurine and D-Glucurono –γ-Lactone as constituents of so-called 'energy' drinks". Directorate-General Health and Consumers, European Commission, European Union. 1999-01-21. http://ec.europa.eu/food/fs/sc/scf/out22_en.html.
- ↑ "Rhodopsin topography and rod-mediated function in cats with the retinal degeneration of taurine deficiency". Experimental Eye Research 45 (4): 481–490. October 1987. doi:10.1016/S0014-4835(87)80059-3. PMID 3428381.
- ↑ "Taurine concentrations in animal feed ingredients; cooking influences taurine content". Journal of Animal Physiology and Animal Nutrition 87 (7–8): 251–262. Aug 2003. doi:10.1046/j.1439-0396.2003.00434.x. PMID 12864905. https://www.vetmed.ucdavis.edu/sites/g/files/dgvnsk491/files/aal/pdfs/spitze.pdf. Retrieved January 27, 2024.
- ↑ "Monosodium glutamate, disodium inosinate, disodium guanylate, lysine and taurine improve the sensory quality of fermented cooked sausages with 50% and 75% replacement of NaCl with KCl". Meat Science 96 (1): 509–513. January 2014. doi:10.1016/j.meatsci.2013.08.024. PMID 24008059.
- ↑ "Taurine in neonatal nutrition – revisited". Archives of Disease in Childhood. Fetal and Neonatal Edition 89 (6): F473–F474. November 2004. doi:10.1136/adc.2004.055095. PMID 15499132.
- ↑ "Taurine in sports and exercise". Journal of the International Society of Sports Nutrition 18 (1). May 2021. doi:10.1186/s12970-021-00438-0. PMID 34039357.
- ↑ "Risk assessment for the amino acids taurine, L-glutamine and L-arginine". Regulatory Toxicology and Pharmacology 50 (3): 376–399. April 2008. doi:10.1016/j.yrtph.2008.01.004. PMID 18325648. "the newer method described as the Observed Safe Level (OSL) or Highest Observed Intake (HOI) was utilized. The OSL risk assessments indicate that based on the available published human clinical trial data, the evidence for the absence of adverse effects is strong for taurine at supplemental intakes up to 3 g/day, glutamine at intakes up to 14 g/day and arginine at intakes up to 20 g/day, and these levels are identified as the respective OSLs for normal healthy adults.".
- ↑ "Safety issues associated with commercially available energy drinks". Journal of the American Pharmacists Association 48 (3): e55–63; quiz e64–7. 2008. doi:10.1331/JAPhA.2008.07055. PMID 18595815.
- ↑ "Retinal Degeneration Associated with Taurine Deficiency in the Cat". Science (New York, N.Y.) 188 (4191): 949–951. 1975. doi:10.1126/science.1138364. PMID 1138364. Bibcode: 1975Sci...188..949H. https://www.science.org/doi/abs/10.1126/science.1138364.
- ↑ Nutrient Requirements of Cats, Revised Edition. Board On Agriculture. 1986. ISBN 978-0-309-07483-4.
- ↑ "Retinal degeneration associated with taurine deficiency in the cat". Science (New York, N.Y.) 188 (4191): 949–951. May 1975. doi:10.1126/science.1138364. PMID 1138364. Bibcode: 1975Sci...188..949H.
- ↑ Morris, J. G.; Rogers, Q. R.; Pacioretty, L. M. (1990). "Taurine: an essential nutrient for cats" (in en). Journal of Small Animal Practice 31 (10): 502–509. doi:10.1111/j.1748-5827.1990.tb00672.x. ISSN 1748-5827. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1748-5827.1990.tb00672.x.
- ↑ "Myocardial failure in cats associated with low plasma taurine: a reversible cardiomyopathy". Science (New York, N.Y.) 237 (4816): 764–768. August 1987. doi:10.1126/science.3616607. PMID 3616607. Bibcode: 1987Sci...237..764P.
- ↑ "AAFCO Cat Food Nutrient Profiles". http://maxshouse.com/nutrition/aafco_cat_food_nutrient_profiles.htm.
- ↑ "The taurine requirement of the adult cat". Journal of Small Animal Practice 23 (9): 533–537. 1982. doi:10.1111/j.1748-5827.1982.tb02514.x.
- ↑ "Clinical significance of taurine". Amino Acids 46 (1): 1–5. January 2014. doi:10.1007/s00726-013-1632-8. PMID 24337931. (abstracts of animal citations used to provide list of species)
- ↑ "Parental prey selection affects risk-taking behaviour and spatial learning in avian offspring". Proceedings. Biological Sciences 274 (1625): 2563–2569. October 2007. doi:10.1098/rspb.2007.0687. PMID 17698490.
- ↑ "Taurine in poultry nutrition". Animal Feed Science and Technology 260. February 2020. doi:10.1016/j.anifeedsci.2019.114339.
- ↑ "Taurine: a critical nutrient for future fish feeds". Aquaculture 437: 215–229. February 2015. doi:10.1016/j.aquaculture.2014.12.006. Bibcode: 2015Aquac.437..215S.
- ↑ "Roles of dietary taurine in fish nutrition". Marine Life Science & Technology 2 (4): 360–375. November 2020. doi:10.1007/s42995-020-00051-1. Bibcode: 2020MLST....2..360S. https://www.researchgate.net/publication/343025001.
- ↑ "Taurine as a constituent of mitochondrial tRNAs: new insights into the functions of taurine and human mitochondrial diseases". The EMBO Journal 21 (23): 6581–6589. December 2002. doi:10.1093/emboj/cdf656. PMID 12456664.
- ↑ Systematic nomenclature of organic, organometallic and coordination chemistry. EPFL Press. 2007. pp. 226. ISBN 978-1-4200-4615-1.
Lua error: Internal error: The interpreter exited with status 1.
Lua error: Internal error: The interpreter exited with status 1. Lua error: Internal error: The interpreter exited with status 1. Lua error: Internal error: The interpreter exited with status 1.
Lua error: Internal error: The interpreter exited with status 1.
 KSF
KSF