Trans,trans,trans-(1,5,9-Cyclododecatriene)nickel(0)
Topic: Chemistry
 From HandWiki - Reading time: 5 min
From HandWiki - Reading time: 5 min
| |
| |
| Names | |
|---|---|
| IUPAC name
(1E,5E,9E)-cyclododeca-1,5,9-triene;nickel
| |
| Other names
all-trans-(1,5,9-Cyclododecatriene)nickel(0), Ni(ttt-cdt)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| Properties | |
| C12H18Ni | |
| Molar mass | 220.96 g/mol |
| Appearance | Red solid |
| Melting point | 140 °C (284 °F; 413 K) (N2, decomposes) |
| Solubility | soluble in diethyl ether |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
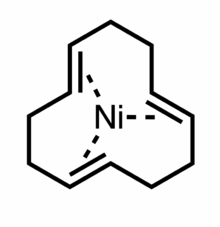
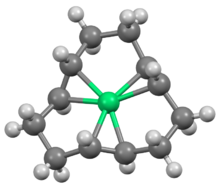
trans,trans,trans-(1,5,9-Cyclododecatriene)nickel(0) a organonickel compound with the formula NiC12H18, better known as t-Ni(cdt). It is a 16-electron coordination complex featuring trigonal planar nickel(0) bound to the three alkene groups in the cyclododecatriene ligand.[1] X-ray structural analysis demonstrates that the three olefins adopt a propeller-like arrangement around the nickel atom center, making the structure chiral.[2] This extremely air-sensitive deep red solid was the first discovered Ni(0)-olefin complex.[3]
Preparation and properties
The complex is prepared by reduction of anhydrous nickel(II) acetylacetonate in ether in the presence of the triolefin:[4]
- Ni(acac)2 + t-cdt + 2 Et2AlOEt → t-Ni(cdt) + 2 acacAlOEt + 2 C2H5•
σ-Donating ligands such as carbon monoxide, isonitriles, phosphines, and hydrides can readily add onto t-Ni(cdt) to furnish tetrahedral 18-electron nickel complexes.[5] It has been demonstrated that this fourth coordination site can be leveraged to separate the t-Ni(cdt) enantiomers with recrystallization of diastereomeric 18-electron t-Ni(cdt)L* complexes (where L* = optically active dimethylmenthylphosphine ligand).[3][4]
Applications
The all-trans-(cdt) ligand has been shown to be easily displaced with olefins such as trans-cyclooctene,[3] ethylene,[6] all-cis-(cdt),[7] norbornene,[6][8] to give the corresponding colorless 16-electron Ni(0)-olefin complexes with coplanar geometry. Ni(cod)2 can also be easily prepared from Ni(cdt).
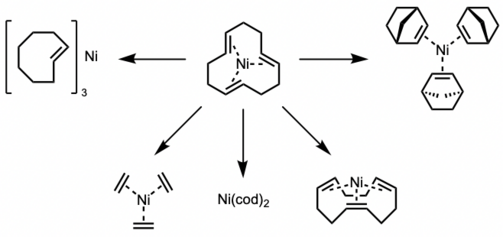
Recently, it was demonstrated that t-Ni(cdt) can be used to synthesize unique air-stable 16-electron Ni(0)–olefin complexes, such as Ni(Fstb)3 and Ni(4-tBustb)3 using (E)-stilbene ligands.[9][10]
References
- ↑ Wilke, G. (1960). "Hauptversammlung der Gesellschaft Deutscher Chemiker". Angewandte Chemie 72 (16): 581–582. doi:10.1002/ange.19600721611.
- ↑ Brauer, D. J.; Krüger, C. (1972). "The three-dimensional structure of trans.trans,trans-1,5,9-cyclododecatrienenickel". Journal of Organometallic Chemistry 44 (2): 397–402. doi:10.1016/S0022-328X(00)82929-0.
- ↑ 3.0 3.1 3.2 Wilke, G. (1988). "Contributions to Organo-Nickel Chemistry". Angewandte Chemie International Edition 27 (1): 185–206. doi:10.1002/anie.198801851.
- ↑ 4.0 4.1 Jolly, P. W. (1974). The Organic Chemistry of Nickel. 1. Elsevier. pp. 252–253. doi:10.1016/B978-0-12-388401-5.X5001-5. ISBN 978-0-12-388401-5.
- ↑ Bogdanović, B.; Kröner, M.; Wilke, G. (1966). "Übergangsmetallkomplexe, I. Olefin-Komplexe des Nickels(0)". Justus Liebigs Annalen der Chemie 699 (1): 1–23. doi:10.1002/jlac.19666990102. PMID 5986842.
- ↑ 6.0 6.1 Fischer, K.; Jonas, K.; Misbach, P.; Stabba, R.; Wilke, G. (1973). "Zum "Nickel-Effekt"︁". Angewandte Chemie 85 (23): 1001–1012. doi:10.1002/ange.19730852302.
- ↑ Jonas, K.; Heimbach, P.; Wilke, G. (1968). "1,5,9-Cyclododecatriene Complexes of Nickel(0)". Angewandte Chemie International Edition 7 (12): 949–950. doi:10.1002/anie.196809491.
- ↑ Lutz, S.; Nattmann, L.; Nöthling, N.; Cornella, J. (2021). "16-Electron Nickel(0)-Olefin Complexes in Low-Temperature C(sp2)–C(sp3) Kumada Cross-Couplings". Organometallics. doi:10.1021/acs.organomet.0c00775. ISSN 0276-7333.
- ↑ Nattman, L.; Saeb, R.; Nöthling, N.; Cornella, P. (2019). "An air-stable binary Ni(0)–olefin catalyst". Nature Catalysis 3 (2020): 6–13. doi:10.1038/s41929-019-0392-6.
- ↑ Nattmann, L.; Cornella, P. (2020). "Ni(4-tBustb)3: A Robust 16-Electron Ni(0) Olefin Complex for Catalysis". Organometallics 39 (18): 3295–3300. doi:10.1021/acs.organomet.0c00485.
 |
 KSF
KSF