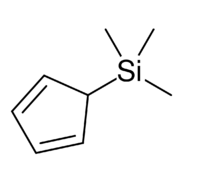
Trimethylsilyl cyclopentadiene
Topic: Chemistry
 From HandWiki - Reading time: 2 min
From HandWiki - Reading time: 2 min
| |
| Names | |
|---|---|
| IUPAC name
cyclopenta-2,4-dien-1-yl(trimethyl)silane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C8H14Si | |
| Molar mass | 138.285 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.833 g/mL[1] |
| Boiling point | 138 to 140 °C (280 to 284 °F; 411 to 413 K)[1] |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H226, H315, H319, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P302+352, P303+361+353, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P370+378, P403+233, P403+235, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Trimethylsilyl cyclopentadiene is an organosilicon compound with the chemical formula C5H5Si(CH3)3. It exists as a colorless liquid. It is used in the synthesis of some metal cyclopentadienyl complexes and has attracted interest for its fluxional structure.
Trimethylsilyl cyclopentadiene is an example of a molecule that undergoes rapid sigmatropic rearrangement. Observations of trimethylsilyl cyclopentadiene using gas phase NMR spectroscopy show that the protons on the ring are chemically equivalent, indicated by a single peak. This phenomenon, an example of fluxionality, is explained by the migration of the silyl group from carbon-to-carbon, thereby giving the appearance of equivalent CH signals.[2]
Properties:[3]
refractive index n20/D 1.471(lit.)
bp 138-140 °C(lit.)
density 0.833 g/mL at 25 °C(lit.)
storage temp. −20 °C
Synthesis
Trimethylsilyl cyclopentadiene is prepared by the reaction trimethylsilyl chloride (Me3SiCl) with sodium cyclopentadienide (NaC5H5):[4][5]
- (CH3)3SiCl + NaC5H5 → C5H5Si(CH3)3 + NaCl
References
- ↑ 1.0 1.1 "Trimethylsilyl cyclopentadiene". Sigma-Aldrich. http://www.sigmaaldrich.com/catalog/product/aldrich/257885?lang=en.
- ↑ Taha, A.N.; Moreno, P.O.; Lemaster, C.B.; Lemaster, C.L.; True, N.S. (2000). "1H NMR observation of trimethylsilyl migration in gas phase 5-trimethylsilylcyclopentadiene". Journal of Molecular Structure 553 (1–3): 37–42. doi:10.1016/S0022-2860(00)00549-4. Bibcode: 2000JMoSt.553...37T.
- ↑ "Trimethylsilyl cyclopentadiene, mixture of isomers 257885". https://www.sigmaaldrich.com/catalog/product/aldrich/257885.
- ↑ Seki, R.; Komatsu, S. (2011). "Preparation of (trialkylsilyl)cyclopentadienes containing almost no bis(trialkylsilyl)cyclopentadienes". Jpn. Kokai Tokkyo Koh..
- ↑ Francisco Palacios; Jesús M. de los Santos (2014). "5-(Trimethylsilyl)-1,3-cyclopentadiene". e-EROS Encyclopedia of Reagents for Organic Synthesis. pp. 1–8. doi:10.1002/047084289X.rn01739. ISBN 978-0-470-84289-8.
 |
 KSF
KSF