Vanadium hexacarbonyl
Topic: Chemistry
 From HandWiki - Reading time: 3 min
From HandWiki - Reading time: 3 min
| |
| Names | |
|---|---|
| IUPAC name
hexacarbonylvanadium(0)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
PubChem CID
|
|
| UNII | |
| |
| Properties | |
| C6O6V | |
| Molar mass | 219.00 g/mol |
| Appearance | blue-green crystals yellow solutions |
| Density | 1.7 g/cm3 |
| Melting point | decomposes |
| Boiling point | sublimes at 50 °C (122 °F; 323 K) (15 mmHg) |
| insoluble | |
| Solubility in other solvents | 5 g/L hexane; more soluble in dichloromethane |
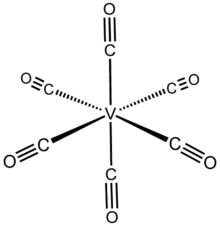
| Structure | |
| orthorhombic | |
| octahedral | |
| 0 D | |
| Hazards | |
| Main hazards | CO source |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Vanadium hexacarbonyl is the inorganic compound with the formula V(CO)6. It is a blue-black volatile solid. This highly reactive species is noteworthy from theoretical perspectives as a rare isolable homoleptic metal carbonyl that is paramagnetic. Most species with the formula Mx(CO)y follow the 18-electron rule, whereas V(CO)6 has 17 valence electrons.[1]
Synthesis
Traditionally V(CO)6 is prepared in two-steps via the intermediacy of V(CO)−6. In the first step, VCl3 is reduced with metallic sodium under 200 atm CO at 160 °C. The solvent for this reduction is typically diglyme, CH3OCH2CH2OCH2CH2OCH3. This triether solubilizes sodium salts, akin to the behavior of a crown ether:
- 4 Na + VCl3 + 6 CO + 2 diglyme → [Na(diglyme)2][V(CO)6] + 3 NaCl
The resulting anion is oxidized with acid:[2]
- 2 V(CO)−6 + 2 H3PO4 → 2 V(CO)6 + H2 + 2 H2PO−4
Reactions
Vanadium hexacarbonyl is thermally unstable. Its primary reaction is reduction to the monoanion V(CO)−6, salts of which are well studied. It is also susceptible to substitution by tertiary phosphine ligands, often leading to disproportionation.
V(CO)6 reacts with sources of the cyclopentadienyl anion to give the orange four-legged piano stool complex (C5H5)V(CO)4 (m.p. 136 °C). Like many charge-neutral organometallic compounds, this half-sandwich species is volatile. In the original preparation of this species, C5H5HgCl was employed as the source of C5H−5.
Structure
V(CO)6 adopts an octahedral coordination geometry and is isostructural with chromium hexacarbonyl, even though they have differing valence electron counts. High resolution X-ray crystallography indicates that the molecule is slightly distorted with two (axial) shorter V–C distances of 1.993(2) Å vs. four (equatorial) 2.005(2) Å. Even though V(−I) is a larger ion than V(0), the V–C distances in V(CO)−6 are 0.07 Å shorter than in the neutral precursor.[3]
References
- ↑ Elschenbroich, C.; Salzer, A. (1992). Organometallics: A Concise Introduction (2nd ed.). Weinheim: Wiley-VCH. ISBN 3-527-28165-7.
- ↑ Liu, X.; Ellis, J. E. (2004). "Hexacarbonylvanadate(1−) and Hexacarbonylvanadium(0)". Inorg. Synth. 34: 96–103. doi:10.1002/0471653683.ch3. ISBN 0-471-64750-0.
- ↑ Bellard, S.; Rubinson, K. A.; Sheldrick, G. M. (1979). "Crystal and Molecular Structure of Vanadium Hexacarbonyl". Acta Crystallographica B35 (2): 271–274. doi:10.1107/S0567740879003332.
Further reading
- Original synthesis: Ercoli, R.; Calderazzo, F.; Alberola, A. (1960). "Synthesis of Vanadium Hexacarbonyl". J. Am. Chem. Soc. 81 (11): 2966–2967. doi:10.1021/ja01496a073.
 |
 KSF
KSF