Vanadium tetrafluoride
Topic: Chemistry
 From HandWiki - Reading time: 4 min
From HandWiki - Reading time: 4 min
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
vanadium tetrafluoride
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| UN number | UN2923 | ||
| |||
| |||
| Properties | |||
| F4V | |||
| Molar mass | 126.9351 g·mol−1 | ||
| Appearance | Lime green powder, hygroscopic[1] | ||
| Odor | Odorless | ||
| Density | 3.15 g/cm3 (20 °C)[1] 2.975 g/cm3 (23 °C)[2] | ||
| Melting point | 325 °C (617 °F; 598 K) at 760 mmHg decomposes[1] | ||
| Boiling point | Sublimes[1] | ||
| Very soluble[1] | |||
| Solubility | Soluble in acetone, acetic acid Very slightly soluble in SO2Cl2, alcohols, CHCl3[2] | ||
| Structure | |||
| Monoclinic, mP10 | |||
| P21/c, No. 14 | |||
| Thermochemistry | |||
Std molar
entropy (S |
126 J/mol·K[3] | ||
Std enthalpy of
formation (ΔfH⦵298) |
−1412 kJ/mol[3] | ||
Gibbs free energy (ΔfG˚)
|
−1312 kJ/mol[3] | ||
| Hazards | |||
| GHS pictograms |  
| ||
| GHS Signal word | Danger | ||
| H300, H330, H314 | |||
| P260, P301+310, P303+361+353, P304+340, P305+351+338, P320, P330, P405, P501 | |||
| Eye hazard | Causes serious damage | ||
| Skin hazard | Causes burns | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tracking categories (test):
Vanadium(IV) fluoride (VF4) is an inorganic compound of vanadium and fluorine. It is paramagnetic yellow-brown solid that is very hygroscopic.[2] Unlike the corresponding vanadium tetrachloride, the tetrafluoride is not volatile because it adopts a polymeric structure.[5] It decomposes before melting.
Preparation and reactions
VF4 can be prepared by treating VCl4 with HF:
- VCl4 + 4 HF → VF4 + 4 HCl
It was first prepared in this way.[6]
It decomposes at 325 °C, undergoing disproportionation to the tri- and pentafluorides:[2]
- 2 VF4 → VF3 + VF5
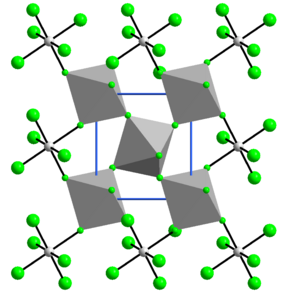
Structure
The structure of VF4 is related to that of SnF4. Each vanadium centre is octahedral, surrounded by six fluoride ligands. Four of the fluoride centers bridge to adjacent vanadium centres.[7]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Lide, David R., ed (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ↑ 2.0 2.1 2.2 2.3 Kwasnik, W. (1963). Brauer, Georg. ed. Handbook of Preparative Inorganic Chemistry (UK ed.). London: Academic Press. pp. 252–253. https://archive.org/details/handbookpreparat01brau_517.
- ↑ 3.0 3.1 3.2 Anatolievich, Kiper Ruslan. "vanadium(IV) fluoride". http://chemister.ru/Database/properties-en.php?dbid=1&id=408.
- ↑ "Vanadium(IV) fluoride, 95%". Alfa Aesar. http://www.alfa.com/en/catalog/11543.
- ↑ Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, p. 716, ISBN 0-471-19957-5
- ↑ Otto Ruff, Herbert Lickfett "Vanadinfluoride" Chemische Berichte 1911, vol. 44, pages 2539–2549. doi:10.1002/cber.19110440379
- ↑ Becker S., Muller B. G. Vanadium Tetrafluoride, Angew. Chem. Intnl. Ed. Engl. 1990, vol. 29, page 406
External links
 |
Licensed under CC BY-SA 3.0 | Source: https://handwiki.org/wiki/Chemistry:Vanadium_tetrafluoride4 views | Status: cached on September 03 2024 07:29:35↧ Download this article as ZWI file
 KSF
KSF