Varacin
Topic: Chemistry
 From HandWiki - Reading time: 2 min
From HandWiki - Reading time: 2 min
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
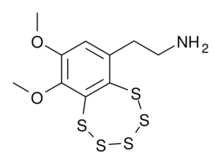
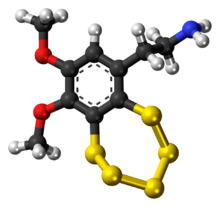
2-(8,9-Dimethoxy-1,2,3,4,5-benzopentathiepin-6-yl)ethan-1-amine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C10H13NO2S5 | |
| Molar mass | 339.540 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Varacin is a bicyclic organosulfur compound originally found in marine Ascidiacea from the Polycitor genus.[1] It contains an unusual pentathiepin ring which reacts with DNA, and varacin and synthetic analogues have been investigated for their antimicrobial and antitumour properties.[2][3] Because of its potent biological activity and unusual and challenging ring system, it has been a popular target of efforts toward its total synthesis.[4][5][6]
References
- ↑ "Varacin and three new marine antimicrobial polysulfides from the far-eastern ascidian Polycitor sp". Journal of Natural Products 58 (2): 254–8. February 1995. doi:10.1021/np50116a015. PMID 7769392.
- ↑ "On the origin of cytotoxicity of the natural product varacin. A novel example of a pentathiepin reaction that provides evidence for a triatomic sulfur intermediate". Journal of the American Chemical Society 123 (42): 10379–86. October 2001. doi:10.1021/ja016495p. PMID 11603989.
- ↑ "The role of amine in the mechanism of pentathiepin (polysulfur) antitumor agents". Journal of the American Chemical Society 125 (2): 396–404. January 2003. doi:10.1021/ja027416s. PMID 12517151.
- ↑ "Total synthesis of the novel benzopentathiepin varacinium trifluoroacetate: the viability of "varacin-free base"". Journal of the American Chemical Society 115 (15): 7017–7018. 1993-07-01. doi:10.1021/ja00068a087. ISSN 0002-7863.
- ↑ "Synthesis and Structural Properties of the Benzopentathiepins Varacin and Isolissoclinotoxin A". The Journal of Organic Chemistry 59 (20): 5955–5960. 1994-10-01. doi:10.1021/jo00099a026. ISSN 0022-3263.
- ↑ "A New Route to the Synthesis of the Naturally Occurring Benzopentathiepin Varacin". Journal of the American Chemical Society 117 (27): 7261–7262. 1995-07-01. doi:10.1021/ja00132a033.
 |
Licensed under CC BY-SA 3.0 | Source: https://handwiki.org/wiki/Chemistry:Varacin13 views | ↧ Download this article as ZWI file
 KSF
KSF