Pfizer
Topic: Company
 From HandWiki - Reading time: 50 min
From HandWiki - Reading time: 50 min
[ ⚑ ] 40°45′01″N 73°58′21″W / 40.75028°N 73.9725°W
 | |
| Type | Public |
|---|---|
| Industry | |
| Founded | 1849 in New York City |
| Founders |
|
| Headquarters | The Spiral, New York City , U.S. |
Area served | Worldwide |
Key people | Albert Bourla (CEO) |
| Products | |
| Revenue | |
| Total assets | |
| Total equity | |
Number of employees | c. 83,000 (2022) |
| Website | pfizer |
| Footnotes / references [1] | |
Pfizer Inc. (/ˈfaɪzər/ FY-zər)[2] is an American multinational pharmaceutical and biotechnology corporation headquartered at The Spiral in Manhattan, New York City . The company was established in 1849 in New York by two German entrepreneurs, Charles Pfizer (1824–1906) and his cousin Charles F. Erhart (1821–1891).
Pfizer develops and produces medicines and vaccines for immunology, oncology, cardiology, endocrinology, and neurology. The company's largest products by sales are the Pfizer–BioNTech COVID-19 vaccine ($37 billion in 2022 revenues), Nirmatrelvir/ritonavir ($18 billion in 2022 revenues), Apixaban ($6 billion in 2022 revenues), a pneumococcal conjugate vaccine ($6 billion in 2022 revenues), and Palbociclib ($5 billion in 2022 revenues).[1] In 2022, 42% of the company's revenues came from the United States, 8% came from Japan, and 50% came from other countries.[1]
Pfizer was a component of the Dow Jones Industrial Average stock market index from 2004 to August 2020.[3][4][5][6] The company ranks 38th on the Fortune 500 [7] and 39th on the Forbes Global 2000.[8]
History
1849–1950: Early history
Pfizer was founded in 1849 as "Charles Pfizer & Company" by Charles Pfizer and Charles F. Erhart,[9] two cousins who had immigrated to the United States from Ludwigsburg, Germany. The business produced chemical compounds, and was headquartered on Bartlett Street[10] in Williamsburgh, New York where they produced an antiparasitic called santonin. This was an immediate success, although it was production of citric acid that led to Pfizer's growth in the 1880s. Pfizer continued to buy property in the area (by now the Williamsburg district of the city of Brooklyn, New York and beginning in 1898, the City of Greater New York) to expand its lab and factory, retaining offices on Flushing Avenue until the 1960s; the Brooklyn plant ultimately closed in 2009.[11] Following their success with citric acid, Pfizer (at the now-demolished 295 Washington Avenue) and Erhart (at 280 Washington Avenue) established their main residences in the nearby Clinton Hill district, known for its concentration of Gilded Age wealth.
In 1881, Pfizer moved its administrative headquarters to 81 Maiden Lane in Manhattan, presaging the company's expansion to Chicago a year later.[10][12] By 1906 sales exceeded $3 million.[13]
World War I caused a shortage of calcium citrate, which Pfizer imported from Italy for the manufacture of citric acid, and the company began a search for an alternative supply. Pfizer chemists learned of a fungus that ferments sugar to citric acid, and they were able to commercialize production of citric acid from this source in 1919. The company developed expertise in fermentation technology as a result. These skills were applied to the deep-submergence mass production of penicillin, an antibiotic, during World War II in response to the need to treat injured Allied soldiers.[14] The company also embarked on a global soil collection program related to improving production yields of penicillin which ultimately resulted in 135,000 samples.[15]
On June 2, 1942, the company incorporated in Delaware.[1]
1950–1980: Pivot to pharmaceutical research and global expansion
Due to price declines for penicillin, Pfizer searched for new antibiotics with greater profit potential. Pfizer discovered oxytetracycline in 1950, and this changed the company from a manufacturer of fine chemicals to a research-based pharmaceutical company. Pfizer developed a drug discovery program focused on in vitro synthesis to augment its research in fermentation technology. In 1959, the company established an animal health division with a 700-acre (2.8 km2) farm and research facility in Terre Haute, Indiana.[14]
By the 1950s, Pfizer had established offices in Belgium, Brazil , Canada , Cuba, Mexico, Panama, Puerto Rico, and the United Kingdom . In 1960, the company moved its medical research laboratory operations out of New York City to a new facility in Groton, Connecticut. In 1980, Pfizer launched Feldene (piroxicam), a prescription anti-inflammatory medication that became Pfizer's first product to reach $1 billion in revenue.[13]
In 1965, John Powers, Jr. became chief executive officer of the company, succeeding John McKeen.[13]
As the area surrounding its Brooklyn plant fell into decline in the 1970s and 1980s, the company formed a public-private partnership with New York City that encompassed the construction of low- and middle-income housing, the refurbishment of apartment buildings for the homeless and the establishment of a charter school.[12]
In 1972, Edmund T. Pratt Jr. became chief executive officer of the company, succeeding John Powers, Jr.[13]
1980–2000: Development of Viagra, Zoloft, and Lipitor
In 1981, the company received approval for Diflucan (fluconazole), the first oral treatment for severe fungal infections including candidiasis, blastomycosis, coccidiodomycosis, cryptococcosis, histoplasmosis, dermatophytosis, and pityriasis versicolor.[16]
In 1986, Pfizer acquired the worldwide rights to Zithromax (azithromycin), a macrolide antibiotic that is recommended by the Infectious Disease Society of America as a first line treatment for certain cases of community-acquired pneumonia, from Pliva.[17][18]
In 1989, Pfizer scientists Peter Dunn and Albert Wood created Viagra (sildenafil) for treating high blood pressure and angina, a chest pain associated with coronary artery disease. In 1991, it was patented in the United Kingdom as a heart medication. Early trials for the medication showed that it did not work for the treatment of heart disease, but volunteers in the clinical trials had increased erections several days after taking the drug. It was patented in the United States in 1996 and received approval by the Food and Drug Administration in March 1998. In December 1998, Pfizer hired Bob Dole as a spokesperson for the drug.[19] The patents for Viagra expired in 2020.[20]
In 1991, William C. Steere, Jr. became chief executive officers of the company, succeeding Edmund T. Pratt Jr.[21]
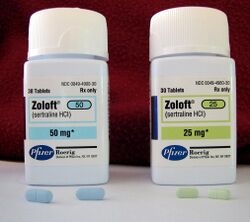
In 1991 Pfizer also began marketing Zoloft (sertraline), an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class developed nine years earlier by Pfizer chemists Kenneth Koe and Willard Welch. Sertraline is primarily prescribed for major depressive disorder in adult outpatients as well as obsessive-compulsive disorder, panic disorder, and social anxiety disorder in both adults and children. In 2005, the year before it became a generic drug, sales were over $3 billion and over 100 million people had been treated with the drug.[22] The patent for Zoloft expired in the summer of 2006.[23]
In 1996, Eisai, in partnership with Pfizer, received approval from the Food and Drug Administration for donepezil under the brand Aricept for treatment of Alzheimer's disease;[24] Pfizer also received approval for Norvasc (amlodipine), an antihypertensive drug of the dihydropyridine calcium channel blocker class.[25]
In 1997, the company entered into a co-marketing agreement with Warner–Lambert for Lipitor (atorvastatin), a statin for the treatment of hypercholesterolemia. Although atorvastatin was the fifth statin to be developed, clinical trials showed that atorvastatin caused a more dramatic reduction in low-density lipoprotein pattern C (LDL-C) than the other statin drugs. Upon its patent expiration in 2011, Lipitor was the best-selling drug ever, with approximately $125 billion in sales over 14.5 years.[26]
2000–2010: Further expansion
In 2001, Henry McKinnell became chief executive officer of the company, replacing William C. Steere, Jr.[27]
In 2002, The Bill & Melinda Gates Foundation purchased stock in Pfizer.[28]
In 2004, the company received approval for Lyrica (pregabalin), an anticonvulsant and anxiolytic medication used to treat epilepsy, neuropathic pain, fibromyalgia, restless leg syndrome, and generalized anxiety disorder.[29][30][31] The United States patent on Lyrica was challenged by generic manufacturers and was upheld in 2014, extending the expiration date to 2018.[32]
In July 2006, Jeff Kindler was named chief executive officer of the company, replacing Henry McKinnell.[27][33]
On December 3, 2006, Pfizer ceased development of torcetrapib, a drug that increases production of HDL, which reduces LDL thought to be correlated to heart disease. During a Phase III clinical trial involving 15,000 patients, more deaths than expected occurred in the group that took the medicine, and the mortality rate of patients taking the combination of torcetrapib and Lipitor (82 deaths during the study) was 60% higher than those taking Lipitor alone (52 deaths during the study). Lipitor alone was not implicated in the results, but Pfizer lost nearly $1 billion developing the failed drug and its stock price dropped 11% on the day of the announcement.[34][35][36][37]
Between 2007 and 2010, Pfizer spent $3.3 million on investigations and legal fees and recovered about $5.1 million, and had another $5 million of pending recoveries from civil lawsuits against makers of counterfeit prescription drugs. Pfizer has hired customs and narcotics experts worldwide to track down fakes and assemble evidence that can be used to pursue civil suits for trademark infringement.[38]
In July 2008, Pfizer announced 275 job cuts at its manufacturing facility in Kalamazoo, Michigan. Kalamazoo was previously the world headquarters of Upjohn Company, which had been acquired as part of Pharmacia.[39][40]
Acquisitions and mergers
In June 2000, Pfizer acquired Warner-Lambert outright for $116 billion. To satisfy conditions imposed by antitrust regulators at the Federal Trade Commission, Pfizer sold off or transferred stakes in several minor products, including RID (a shampoo for treatment of head lice, sold to Bayer) and Warner-Lambert's antidepressant Celexa (which competes with Zoloft).[41] The acquisition created what was, at the time, the second-largest pharmaceutical company worldwide.[42]
In 2003, Pfizer merged with Pharmacia, and in the process acquired Searle and SUGEN. Searle had developed Flagyl (metronidazole), a nitroimidazole antibiotic medication used particularly for anaerobic bacteria and protozoa.[40][43] Searle also developed celecoxib (Celebrex) a COX-2 inhibitor and nonsteroidal anti-inflammatory drug (NSAID) used to treat the pain and inflammation in osteoarthritis, acute pain in adults, rheumatoid arthritis, ankylosing spondylitis, painful menstruation, and juvenile rheumatoid arthritis.[44] SUGEN, a company focused on protein kinase inhibitors, had pioneered the use of ATP-mimetic small molecules to block signal transduction. The SUGEN facility was shut down in 2003 by Pfizer, with the loss of more than 300 jobs, and several programs were transferred to Pfizer. These included sunitinib (Sutent), a cancer medication which was approved for human use by the FDA in January 2006.[45][46] A related compound, SU11654 (Toceranib), was also approved for cancer in dogs, and the ALK inhibitor Crizotinib also grew out of a SUGEN program.[47][48]
In October 2006, the company announced it would acquire PowerMed.[49]
On October 15, 2009, Pfizer acquired Wyeth for $68 billion in cash and stock, including the assumption of debt, making Pfizer the largest pharmaceutical company in the world.[50][51][52][53][54] The acquisition of Wyeth provided Pfizer with a pneumococcal conjugate vaccine, trademarked Prevnar 13; this is used for the prevention of invasive pneumococcal infections. The introduction of the original, 7-valent version of the vaccine, developed by Wyeth in February 2000, led to a 75% reduction in the incidence of invasive pneumococcal infections among children under age 5 in the United States. Pfizer introduced an improved version of the vaccine in 2010, for which it was granted a patent in India in 2017. Prevnar 13 provides coverage of 13 bacterial variants, expanding beyond the original 7-valent version.[54] By 2012, the rate of invasive infections among children under age 5 had been reduced by an additional 50%.[55][56]
2010–2020: Further discoveries and acquisitions

In 2010, Ian Read was named chief executive officer of the company.[58]
In February 2011, Pfizer announced the closure of its UK research and development facility (formerly also a manufacturing plant) in Sandwich, Kent, which at the time employed 2,400 people.[59] In March 2011, Pfizer acquired King Pharmaceuticals for $3.6 billion in cash. King produced emergency injectables such as the EpiPen.[60]
On September 4, 2012, the FDA approved bosutinib (Bosulif) for chronic myelogenous leukemia (CML), a rare type of leukemia and a blood and bone marrow disease that affects primarily older adults.[61] In November 2012, Pfizer received approval from the Food and Drug Administration for Xeljanz, a tofacitinib, for rheumatoid arthritis and ulcerative colitis.[62] The drug had sales of $1.77 billion in 2018, and in January 2019, it was the top drug in the United States for direct-to-consumer advertising, passing adalimumab (Humira).[63]
On February 1, 2013, Zoetis, the Agriculture Division of Pfizer and later Pfizer Animal Health, became a public company via an initial public offering, raising $2.2 billion.[64][65][66][67] Later in 2013, Pfizer completed the corporate spin-off of its remaining stake in Zoetis.[68][69]
In September 2014, the company acquired Innopharma for $225 million, plus up to $135 million in milestone payments, in a deal that expanded Pfizer's range of generic and injectable drugs.[70][71]
On January 5, 2015, the company announced it would acquire a controlling interest in Redvax, expanding its vaccine portfolio targeting human cytomegalovirus.[72] In February 2015, the company received approval from the Food and Drug Administration for palbociclib (Ibrance) for treatment of certain types of breast cancer.[73][74] In March 2015, the company announced it would restart its collaboration with Eli Lilly and Company surrounding the Phase III trial of Tanezumab.[75] In May 2015, Pfizer and a Bar-Ilan University laboratory announced a partnership based on the development of medical DNA nanotechnology.[76] In June 2015, the company acquired Nimenrix and Mencevax, meningococcal vaccines, from GlaxoSmithKline for around $130 million.[77] In September 2015, Pfizer acquired Hospira for $17 billion, including the assumption of debt.[78][79][80][81][82][83] Hospira was the largest producer of generic injectable pharmaceuticals in the world.[84] On November 23, 2015, Pfizer and Allergan announced a planned $160 billion merger, in the largest pharmaceutical deal ever and the third largest corporate merger in history. The proposed transaction contemplated that the merged company maintain Allergan's Republic of Ireland domicile, resulting in the new company being subject to corporation tax at the relatively low rate of 12.5%.[85] The deal was to constitute a reverse merger, whereby Allergan acquired Pfizer, with the new company then changing its name to "Pfizer, plc".[86][87][88] On April 6, 2016, Pfizer and Allergan terminated the merger agreement after the Obama administration and the United States Department of the Treasury introduced new laws intended to limit corporate inversions (the extent to which companies could move their headquarters overseas in order to reduce the amount of taxes they pay).[89][90]
In June 2016, the company acquired Anacor Pharmaceuticals for $5.2 billion, expanding its portfolio in both inflammation and immunology drugs areas.[91][92] In August 2016, the company made a $40 million bid for the assets of BIND Therapeutics, which was in bankruptcy.[93] The same month, the company acquired Bamboo Therapeutics for $645 million, expanding its gene therapy offerings.[94] In September 2016, the company acquired cancer drug-maker Medivation for $14 billion.[95][96][97] In October 2016, the company licensed the anti-CTLA4 monoclonal antibody, ONC-392, from OncoImmune.[98][99] In November 2016, Pfizer funded a $3,435,600 study with the CDC Foundation to research "screen-and-treat" strategies for cryptococcal disease in Botswana.[100] In December 2016, Pfizer acquired AstraZeneca's small-molecule antibiotics business for $1.575 billion.[101][102][103]
In January 2018, Pfizer announced that it would end its work on research into treatments for Alzheimer's disease and Parkinsonism (a symptom of Parkinson's disease and other conditions). The company said about 300 researchers would lose their jobs.[104] In July 2018, the Food and Drug Administration approved enzalutamide, developed by Pfizer and Astellas Pharma for patients with castration-resistant prostate cancer.[105] In August 2018, Pfizer signed an agreement with BioNTech to conduct joint research and development activities regarding mRNA-based influenza vaccines.[106] In October 2018, effective January 1, 2019, Albert Bourla was promoted to chief executive officer, succeeding Ian Read, his mentor.[107][108][109][110]
In July 2019, the company acquired Therachon for up to $810 million, expanding its rare disease portfolio through Therachon's recombinant human fibroblast growth factor receptor 3 compound, aimed at treating conditions such as achondroplasia.[111] Also in July, Pfizer acquired Array Biopharma for $10.6 billion, boosting its oncology pipeline.[112] In August 2019, Pfizer merged its consumer health business with that of GlaxoSmithKline, into a joint venture owned 68% by GlaxoSmithKline and 32% by Pfizer, with plans to make it a public company. The transaction built on a 2018 transaction where GlaxoSmithKline acquired Novartis' stake in the GSK-Novartis consumer healthcare joint business.[113] The transaction followed negotiations with other companies including Reckitt Benckiser,[114] Sanofi, Johnson & Johnson,[115] and Procter & Gamble.[116] In September 2019, Pfizer initiated a study with the CDC Foundation to investigate the tracking of healthcare-associated infections, scheduled to run through to June 2023.[100] In December 2019, Pfizer awarded the CDC Foundation a further $1,948,482 to continue its cryptococcal disease screening and treatment research in nine African countries.[100]
In September 2020, the company acquired a 9.9% stake in CStone Pharmaceuticals for $200 million (HK$1.55 billion), helping to commercialise its anti-PD-L1 monoclonal antibody, CS1001.[117] In October 2020, the company acquired Arixa Pharmaceuticals.[118] In November 2020, using a Reverse Morris Trust structure, Pfizer merged its off-patent branded and generic drug business, known as Upjohn, with Mylan to form Viatris, owned 57% by Pfizer shareholders.[119][120]
2021–onwards: Corporate developments and acquisitions
On January 5, 2021, Pfizer introduced a new logo.[121] In April 2021, Pfizer acquired Amplyx Pharmaceuticals and its anti-fungal compound fosmanogepix (APX001).[122][123] In August, the company announced it would acquire Trillium Therapeutics Inc and its immuno-oncology portfolio for $2.3 billion.[124][125]
In March 2022, the company acquired Arena Pharmaceuticals for $6.7 billion in cash.[126][127][128] In June 2022, the company acquired ReViral Ltd, for up to $525 million, gaining access to experimental drugs used to combat respiratory syncytial virus infections.[129][130] In October 2022, the company acquired Biohaven Pharma and its calcitonin gene-related peptide programs for $11.6 billion.[131][132][133] It also acquired Global Blood Therapeutics for $5.4 billion, boosting Pfizer's rare disease business.[134][135][136]
In March 2023, the company agreed to acquire Seagen for a total enterprise value of around $43 billion.[137] In this regard, the European Commission is conducting an antitrust investigation, which is necessary for the approval of the transaction.[138] On April 3, 2023, Pfizer announced that it had moved its world headquarters from its longtime home of six decades on 42nd Street in Midtown Manhattan to the Spiral at Hudson Yards.[139][57] In the same summer, Pfizer's important injectables manufacturing facility in Rocky Mount, North Carolina was hit by a tornado and had to close production for several weeks.[140]
Acquisition history
- Pfizer (Founded 1849 as Charles Pfizer & Company)
- Warner–Lambert
- William R. Warner (Founded 1856, merged 1955)
- Lambert Pharmacal Company (Merged 1955)
- Parke-Davis (Founded 1860, Acq 1976)
- Wilkinson Sword (Acq 1993, divested 2003)
- Agouron (Acq 1999)
- Pharmacia (Acq 2002)
- Pharmacia & Upjohn (Merged 2000)
- Pharmacia (Merged 1995)
- Farmitalia Carlo Erba
- Kabi Pharmacia
- Pharmacia Aktiebolaget
- The Upjohn Company (Merged 1995)
- Monsanto (Merged 2000, divested 2002)
- Searle (Merged 2000)
- Pharmacia (Merged 1995)
- Pharmacia & Upjohn (Merged 2000)
- Esperion Therapeutics (Acq 2003, divested 2008)
- Meridica (Acq 2004)
- Vicuron Pharmaceuticals (Acq 2005)
- Idun (Acq 2005)
- Angiosyn (Acq 2005)
- Powermed (Acq 2006)
- Rinat (Acq 2006)
- Coley Pharmaceutical Group (Acq 2007)
- CovX (Acq 2007)
- Encysive Pharmaceuticals Inc (Acq 2008)
- Wyeth (Acq 2009)
- Chef Boyardee (Acq 1946, divested 1996 with food div)
- S.M.A. Corporation
- Ayerst Laboratories (Acq 1943)
- Fort Dodge Serum Company (Acq 1945)
- Bristol-Myers (Animal Health div)
- Parke-Davis (Animal Health div)
- A.H. Robins
- Sherwood Medical (Acq 1982)
- Genetics Institute, Inc. (Acq 1992)
- American Cyanamid (Acq 1994)
- Lederle Laboratories
- Solvay (Acq 1995, Animal Health div)
- King Pharmaceuticals (Acq 2010)
- Monarch Pharmaceuticals, Inc.
- King Pharmaceuticals Research and Development, Inc.
- Meridian Medical Technologies, Inc.
- Parkedale Pharmaceuticals, Inc.
- King Pharmaceuticals Canada Inc.
- Monarch Pharmaceuticals Ireland Limited
- Synbiotics Corporation (Acq 2011)
- Icagen (Acq 2011)
- Ferrosan (Consumer Health div, Acq 2011)
- Excaliard Pharmaceuticals (Acq 2011)
- Alacer Corp (Acq 2012)
- NextWave Pharmaceuticals, Inc (Acq 2012)
- Innopharma (Acq 2014)
- Redvax GmbH (Acq 2014)
- Hospira (Spun off from Abbott Laboratories 2004, Acq 2015)
- Mayne Pharma Ltd (Acq 2007)
- Pliva-Croatia
- Orchid Chemicals & Pharmaceuticals Ltd. (Generics & Injectables div, Acq 2009)
- Javelin Pharmaceuticals, Inc. (Acq 2010)
- TheraDoc (Acq 2010)
- Arixa Pharmaceuticals (Acq 2020)
- Anacor Pharmaceuticals(Acq 2016)
- Bamboo Therapeutics (Acq 2016)
- Medivation (Acq 2016)
- AstraZeneca (Small molecule antibiotic div, Acq 2016)
- Array BioPharma (Acq 2019)
- Amplyx Pharmaceuticals (Acq 2021)
- Trillium Therapeutics (Acq 2021)
- Arena Pharmaceuticals (Acq 2022)
- ReViral Ltd (Acq 2022)
- Biohaven Pharma (Acq 2022)
- Kleo Pharmaceuticals, Inc. (Acq 2021)
- Seagen (Acq 2023)
- Cascadian Therapeutics (Acq 2018)
- Warner–Lambert
COVID-19
Pfizer has developed and launched several products in response to the COVID-19 pandemic, including the Pfizer–BioNTech COVID-19 vaccine and Paxlovid.
In March 2020, Pfizer joined the COVID-19 Therapeutics Accelerator funding vehicle to expedite development of treatments against COVID-19.[141][142] The $125 million initiative was launched by the Bill & Melinda Gates Foundation in partnership with Mastercard and Wellcome Trust, with additional funding announced shortly after from Chan Zuckerberg Initiative, UK Foreign, Commonwealth and Development Office and Madonna.[143][144]
The following month, the Foundation for the National Institutes of Health announced the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) public-private partnership to develop a coordinated research strategy for prioritizing and speeding up development of COVID-19 vaccines and pharmaceutical products.[145] Pfizer joined the partnership as an industry "leadership organization", and participated as a collaborator in ACTIV-led clinical trials.[146][147] CEO Albert Bourla attended the GAVI COVAX AMC 2021 Investment Opportunity Launch Event, otherwise named One World Protected, on April 15, 2021.[148]
In Canada, Pfizer endorsed the use of a vaccine passport mobile app developed by CANImmunize in order to record and track status of COVID-19 vaccination.[149]
COVID-19 pandemic and vaccine development
Initial development and testing
As the scale of the COVID-19 pandemic became apparent, Pfizer partnered with BioNTech to study and develop COVID-19 mRNA vaccine candidates. Unlike many of its competitors, Pfizer took no initial research funds from the United States' Operation Warp Speed vaccine development program, instead choosing to invest roughly $2 billion of its own funds. Pfizer CEO Albert Bourla has said that he declined money from Operation Warp Speed to avoid government intervention, stating later that "when you get money from someone that always comes with strings. They want to see how we are going to progress, what type of moves you are going to do. They want reports. And also, I wanted to keep Pfizer out of politics, by the way."[150]
In May 2020, Pfizer began testing four different COVID-19 vaccine variations using lipid nanoparticle technology provided by Canadian biotechnology company Acuitas Therapeutics.[151] Vaccines were injected into the first human participants in the U.S. in early May. In July 2020, Pfizer and BioNTech announced that two of the partners' four mRNA vaccine candidates had won fast track designation from the FDA.[152] The company began Phase II-III testing on 30,000 people in the last week of July 2020 and was slated to be paid $1.95 billion for 100 million doses of the vaccine by the US government.[153] In September 2020, Pfizer and BioNTech announced that they had completed talks with the European Commission to provide an initial 200 million vaccine doses to the EU, with the option to supply another 100 million doses at a later date.[154]
Efficacy results and authorization
On November 9, 2020, Pfizer announced that BioNTech's COVID-19 vaccine, tested on 43,500 people, was found to be 90% effective at preventing symptomatic COVID-19.[155] The efficacy was updated to 95% a week later.[156] Akiko Iwasaki, an immunologist interviewed by the New York Times, described the efficacy figure as "really a spectacular number."[157] The announcement made Pfizer and BioNTech the first companies to develop and test a working vaccine for COVID-19.[156]
Over the following month and a half, regulators in various countries approved Pfizer's vaccine for emergency use. The United Kingdom approved the vaccine first, on December 2,[158] followed by Bahrain on December 4,[159] Canada on December 9,[160] and Saudi Arabia on December 10.[161] On December 10, 2020, the United States FDA held an advisory committee meeting to discuss authorization of the vaccine.[162] The next day, the US officially became the 5th country to approve use of Pfizer's vaccine under an Emergency Use Authorization (EUA), with an independent panel voting 17–4 in support of approval.[162][163] On December 14, Singapore became one of the first in Asia to approve the vaccine through the Health Sciences Authority.[164] On December 21, the European Medicines Agency (EMA) recommended granting a conditional marketing authorisation for the vaccine in the European Union, under the brand name "Comirnaty."[165]
Manufacturing and distribution
As of early May 2021, Pfizer and BioNTech had manufactured at least 430 million vaccine doses, which have been distributed to 91 countries and territories. The companies have said they expect to manufacture nearly 3 billion total vaccine doses in 2021.[166] Pfizer provided 0.9% Sodium chloride Injection USP diluent for use with the Pfizer–BioNTech COVID-19 vaccine under the name of its subsidiary, Hospira.[167] Pfizer also purchased large quantities of single-use medical and surgical gloves and protective bodysuits from Ansell during the process, contributing to a doubling of the supplier's manufacturing capacity.[168]
Controversy
In February 2021, after a year long investigation relying on unnamed officials, Pfizer was accused by The Bureau of Investigative Journalism (TBIJ) of employing "high-level bullying" against at least two Latin American countries during negotiations to acquire COVID-19 vaccines, including requesting that the countries put sovereign assets as collateral for payments.[169] According to TBIJ, these negotiation tactics resulted in a months long delay in Pfizer reaching a vaccine agreement with one country and a complete failure to reach agreements with two other countries, including Argentina and Brazil.[169]
On 2 November 2021, TBMJ published an article after obtaining information from a whistleblower from the Ventavia Research Group. Ventavia was hired by Pfizer as a research subcontractor. A regional director (whistleblower) who was employed at Ventavia Research Group has told The BMJ that the company falsified data, unblinded patients, employed inadequately trained vaccinators, and was slow to follow up on adverse events reported in Pfizer's pivotal phase III trial. The regional director, Brook Jackson, emailed a complaint to the US Food and Drug Administration (FDA). Ventavia fired her later the same day.[170] The European Medicines Agency (EMA) stated in a response to the European Parliament, that "the deficiencies identified do not jeopardize the quality and integrity of the data from the main Comirnaty trial and have no impact on the benefit-risk assessment or on the conclusions on the safety, effectiveness and quality of the vaccine".[171] Science-Based Medicine emphasized that Ventavia oversaw just three of the 153 clinical sites involved with Pfizer's trial and "a small fraction (~1,000 by the time the whistleblower was fired) of the trial's over ~44,000 subjects."[172]
On 10 October 2022, during a session of the European Parliament's Special Committee on the COVID-19 Pandemic, Pfizer executive Janine Small testified that the company had not evaluated their COVID-19 vaccine for its ability to reduce transmission of the SARS-CoV-2 virus prior to its release to the general public.[173][174] Dutch MEP Rob Roos described the admission as "scandalous".[175] CEO Albert Bourla was slated to attend, but withdrew.[176] Roos' statements in turn have been described as "misleading".[177]
Development of oral antivirals
In November 2021, Pfizer launched a new COVID-19 oral antivirus treatment known as Paxlovid. In January 2022, the Pfizer CEO Albert Bourla confirmed that the trial results of a fourth dose were pending until March 2022. He said that the firm was setting up a collaboration to develop an anti-COVID pill treatment along with a French company, Novasep. He also said the COVID vaccine was "safe and efficient" for children.[178][179] In May 2022, reports emerged of patients experiencing "rebound" symptoms after completing a five-day course of Paxlovid.[180] The FDA responded by announcing they had performed additional analyses of the drug's clinical trial data, and decided against changing its recommendations.[181] U.S. President Joe Biden and Dr. Anthony Fauci were both reported to experience this rebound syndrome in the months that followed, while continuing to recommend the drug for those who may benefit from it.[182]
Legal issues
Aggressive pharmaceutical marketing
Pfizer has been accused of aggressive pharmaceutical marketing.[183][184][185]
Illegal marketing of gabapentin for off-label uses
In 1993, the Food and Drug Administration (FDA) approved gabapentin only for treatment of seizures. Warner–Lambert, which merged with Pfizer in 2000, used continuing medical education and medical research, sponsored articles about the drug for the medical literature, and alleged suppression of unfavorable study results, to promote gabapentin. Within five years, the drug was being widely used for off-label uses such as treatment of pain and psychiatric conditions. Warner–Lambert admitted to violating FDA regulations by promoting the drug for pain, psychiatric conditions, migraine, and other unapproved uses.[186] In 2004, the company paid $430 million in one of the largest settlements to resolve criminal and civil health care liability charges. It was the first off-label promotion case successfully brought under the False Claims Act.[187] A Cochrane review concluded that gabapentin is ineffective in migraine prophylaxis.[188] The American Academy of Neurology rates it as having unproven efficacy, while the Canadian Headache Society and the European Federation of Neurological Societies rate its use as being supported by moderate and low-quality evidence.[189]
Illegal marketing of Bextra
In September 2009, Pfizer pleaded guilty to the illegal marketing of arthritis drug valdecoxib (Bextra) and agreed to a $2.3 billion settlement, the largest health care fraud settlement at that time.[190] Pfizer promoted the sale of the drug for several uses and dosages that the Food and Drug Administration specifically declined to approve due to safety concerns. The drug was pulled from the market in 2005.[191] It was Pfizer's fourth such settlement in a decade.[192][193][194] The payment included $1.195 billion in criminal penalties for felony violations of the Federal Food, Drug, and Cosmetic Act, and $1.0 billion to settle allegations it had illegally promoted the drugs for uses that were not approved by the Food and Drug Administration (FDA) leading to violations under the False Claims Act as reimbursements were requested from Federal and State programs. The criminal fine was the largest ever assessed in the United States to date.[192][193][194] Pfizer entered a corporate integrity agreement with the Office of Inspector General that required it to make substantial structural reforms within the company, and publish to its website its post approval commitments and a searchable database of all payments to physicians made by the company.[195]
Termination of Peter Rost
Peter Rost was vice president in charge of the endocrinology division at Pharmacia before its acquisition by Pfizer. During that time he raised concerns internally about kickbacks and off-label marketing of Genotropin, Pharmacia's human growth hormone drug. Pfizer reported the Pharmacia marketing practices to the FDA and Department of Justice; Rost was unaware of this and filed an FCA lawsuit against Pfizer. Pfizer kept him employed, but isolated him until the FCA suit was unsealed in 2005. The Justice Department declined to intervene, and Pfizer fired him, and he filed a wrongful termination suit against Pfizer. Pfizer won a summary dismissal of the case, with the court ruling that the evidence showed Pfizer had decided to fire Rost prior to learning of his whistleblower activities.[196][197]
Illegal marketing of Rapamune
A "whistleblower suit" was filed in 2005 against Wyeth, which was acquired by Pfizer in 2009, alleging that the company illegally marketed sirolimus (Rapamune) for off-label uses, targeted specific doctors and medical facilities to increase sales of Rapamune, tried to get transplant patients to change from their transplant drugs to Rapamune, and specifically targeted African-Americans. According to the whistleblowers, Wyeth also provided doctors and hospitals that prescribed the drug with kickbacks such as grants, donations, and other money.[198] In 2013, the company pleaded guilty to criminal mis-branding violations under the Federal Food, Drug, and Cosmetic Act. By August 2014, it had paid $491 million in civil and criminal penalties related to Rapamune.[199]
Illegal marketing
In June 2010, health insurance network Blue Cross Blue Shield (BCBS) filed a lawsuit against Pfizer for allegedly illegally marketing drugs Bextra, Geodon and Lyrica. BCBS alleged that Pfizer used kickbacks and wrongly persuaded doctors to prescribe the drugs.[200][201] According to the lawsuit, Pfizer handed out 'misleading' materials on off-label uses, sent over 5,000 doctors on trips to the Caribbean or around the United States, and paid them $2,000 honoraria in return for listening to lectures about Bextra.[202][203] Despite Pfizer's claims that "the company's intent was pure" in fostering a legal exchange of information among doctors, an internal marketing plan revealed that Pfizer intended to train physicians "to serve as public relations spokespeople."[204] The case was settled in 2014 for $325 million.[205] Fearing that Pfizer is "too big to fail" and that prosecuting the company would result in disruptions to Medicare and Medicaid, federal prosecutors instead charged a subsidiary of a subsidiary of a subsidiary of Pfizer, which is "nothing more than a shell company whose only function is to plead guilty."[204]
Quigley Company asbestos
The Quigley Company, which sold asbestos-containing insulation products until the early 1970s, was acquired by Pfizer in 1968. In June 2013, asbestos victims and Pfizer negotiated a settlement that required Pfizer to pay a total of $964 million: $430 million to 80% of existing plaintiffs and place an additional $535 million into a settlement trust that will compensate future plaintiffs as well as the remaining 20% of plaintiffs with claims against Pfizer and Quigley. Of that $535 million, $405 million is in a 40-year note from Pfizer, while $100 million is from insurance policies.[206]
Shiley defective heart valves
Pfizer purchased Shiley in 1979, at the onset of its Convexo-Concave valve ordeal, involving the Bjork–Shiley valve. Approximately 500 people died when defective heart valves fractured and, in 1994, Pfizer agreed to pay $10.75 million to settle claims by the United States Department of Justice that the company lied to get approval for the valves.[207]
Firing of employee that filed suit
A federal lawsuit was filed by a scientist claiming she got an infection by a genetically modified lentivirus while working for Pfizer, resulting in intermittent paralysis.[208] A judge dismissed the case citing a lack of evidence that the illness was caused by the virus but the jury ruled that by firing the employee, Pfizer violated laws protecting freedom of speech and whistleblowers and awarded her $1.37 million.[209]
Celebrex intellectual property
Brigham Young University (BYU) said a professor of chemistry, Dr. Daniel L. Simmons, discovered an enzyme in the 1990s that led towards development of Celebrex. BYU was originally seeking a 15% royalty on sales, equating to $9.7 billion. A research agreement had been made between BYU and Monsanto, whose pharmaceutical business was later acquired by Pfizer, to develop a better aspirin. The enzyme Dr. Simmons claims to have discovered would induce pain and inflammation while causing gastrointestinal problems and Celebrex is used to reduce those issues. A six-year battle ensued because BYU claimed that Pfizer did not give Dr. Simmons credit or compensation, while Pfizer claimed that it had met all obligations regarding the Monsanto agreement. In May 2012, Pfizer settled the allegations, agreeing to pay $450 million.[210]
Nigeria Trovafloxacin lawsuit
In 1996, an outbreak of measles, cholera, and bacterial meningitis occurred in Nigeria. Pfizer representatives and personnel from a contract research organization (CRO) traveled to Kano to set up a clinical trial and administer an experimental antibiotic, trovafloxacin, to approximately 200 children.[211] Local Kano officials reported that more than fifty children died in the experiment, while many others developed mental and physical deformities.[212] The nature and frequency of both fatalities and other adverse outcomes were similar to those historically found among pediatric patients treated for meningitis in sub-Saharan Africa.[213] In 2001, families of the children, as well as the governments of Kano and Nigeria, filed lawsuits regarding the treatment.[214] According to Democracy Now!, "[r]esearchers did not obtain signed consent forms, and medical personnel said Pfizer did not tell parents their children were getting the experimental drug."[215] The lawsuits also accused Pfizer of using the outbreak to perform unapproved human testing, as well as allegedly under-dosing a control group being treated with traditional antibiotics in order to skew the results of the trial in favor of Trovan. Nigerian medical personnel as well as at least one Pfizer physician said the trial was conducted without regulatory approval.[216][217]
In 2007, Pfizer published a Statement of Defense letter, stating that the drug's oral form was safer and easier to administer. Trovan had been used safely in more than five thousand Americans prior to the Nigerian trial, and mortality in the patients treated by Pfizer was lower than that observed historically in African meningitis epidemics. No unusual side effects, unrelated to meningitis, were observed after four weeks.[218]
In June 2010, the US Supreme Court rejected Pfizer's appeal against a ruling allowing lawsuits by the Nigerian families to proceed.[219]
In December 2010, the United States diplomatic cables leak indicated that Pfizer hired investigators to find evidence of corruption against Nigerian attorney general Michael Aondoakaa to persuade him to drop legal action.[220] The Washington Post reporter Joe Stephens, who helped break the story in 2000, called these actions "dangerously close to blackmail".[215] In response, the company released a press statement describing the allegations as "preposterous" and saying that it acted in good faith.[221] Aondoakka, who had allegedly demanded bribes from Pfizer in return for a settlement of the case,[222] was declared unfit for office and had his U.S. visa revoked in association with corruption charges in 2010.[223][224]
The lawsuits were eventually settled out of court. Pfizer committed to paying US$35 million "to compensate the families of children in the study", another US$30 million to "support healthcare initiatives in Kano", and $10 million to cover legal costs. Payouts began in 2011.[225]
Inflating Prices
In July 2022, UK antitrust authorities fined Pfizer £63 million for unfairly high priced drug that aids in controlling epileptic seizures. The Competition and Markets Authority stated that the company took advantage of loopholes by de-branding epilepsy drug Epanutin, by doing so the price of Epanutin's price was not regulated to the same standards the company are used to and therefore the price of the drug was raised. It was stated that over a four-year period, Pfizer had billed Epanutin for around 780% and 1,600% higher than its standard price.[226]
Allegations of patent infringement on mRNA technology
In August 2022, Moderna announced that it will sue Pfizer and its partner BioNTech for infringing their patent on the mRNA technology.[227]
Environmental record
Since 2000, the company has implemented more than 4,000 greenhouse gas reduction projects.[228]
In 2012, the company was named to the Carbon Disclosure Project's Carbon Leadership Index in recognition of its efforts to reduce greenhouse gas emissions.[229]
Pfizer has inherited Wyeth's liabilities in the American Cyanamid site in Bridgewater Township, New Jersey, a highly toxic EPA Superfund site. Pfizer has since attempted to remediate this land in order to clean and develop it for future profits and potential public uses.[230] The Sierra Club and the Edison Wetlands Association have opposed the cleanup plan, arguing that the area is subject to flooding, which could cause pollutants to leach. The EPA considers the plan the most reasonable from considerations of safety and cost-effectiveness, arguing that an alternative plan involving trucking contaminated soil off site could expose cleanup workers. The EPA's position is backed by the environmental watchdog group CRISIS.[231]
In June 2002, a chemical explosion at the Groton plant injured 7 people and caused the evacuation of more than 100 homes in the surrounding area.[232]
Public-private engagement
Pfizer engages with the public and private sectors in a variety of settings including to promote research and development, academic funding, event sponsorship, philanthropy, and political lobbying.
Academia
- Institute for Advanced Study - Matching gifts and direct donor.[233][234]
- University of Toronto - Donor to the Boundless Campaign,[235] and member of the President's Circle.[236]
- University of Washington - Member of the Honor Roll of Donors, having contributed between $10 million and $50 million to funding the school as of 2020.[237]
Activism
- Habitat for Humanity - Donor.[238]
- Human Rights Campaign (HRC) - Corporate partner.[239] HRC is a large LGBT civil rights activism group.
- National Women's Law Center - Donor.[240]
- Share Our Strength - Donor.[241]
- WaterAid - Partner.[242]
Conferences and summits
- Women in Medicine Summit - Sponsor.[243]
- World Neuroscience Innovation Forum - Strategic partner.[244]
Media
During the COVID-19 pandemic, Pfizer engaged many forms of media to promote their COVID-19 vaccine, including a commissioned National Geographic documentary.[245] Pfizer is also a donor to the National Geographic Society.[246]
Pfizer was a prominent sponsor of the 2022 Oscars ceremony alongside BioNTech.[247]
Pfizer has been a major donor to the National Press Foundation.[248][249] Pfizer sponsored a program for the NPF called "Cancer Issues 2010" to train journalists to "understand the latest research" on various cancers, including the role of pharmaceutical products and vaccines. MicroRNA (miRNA) was also a listed topic.[250][251]
Pfizer sponsors 19 to Zero, a "coalition of academics, public health experts, behavioural economists, and creative professionals" that develops media and educational materials to influence public perception surrounding COVID-19 and COVID-19 vaccines.[252]
Medical societies
- American Society of Hematology - Sponsor.[253]
- Arthritis Society - National partner. Pfizer also supports the organization's provincial branches in Alberta, British Columbia, Manitoba, New Brunswick, Newfoundland and Labrador, Nova Scotia, Ontario, Prince Edward Island, and Quebec.[254]
- Canadian Cancer Society - Sponsor.[255]
- Canadian Paediatric Society - Funding. CPS is the organization that administers the Canadian Immunization Monitoring Program, Active (IMPACT) vaccine safety program.[256]
- Canadian Society of Internal Medicine - Annual conference sponsor with Bristol Myers Squibb.[257]
- Endocrine Society - Corporate Liaison Board member.[258]
- European Society of Cardiology - Sponsor of the EURObservational Research Programme.[259]
- Spanish Cardiac Society - Strategic partner.[260]
Political lobbying
Pfizer is affiliated with a variety of industry organizations engaging in political lobbying, and has made substantial direct donations to government and regulatory agencies:
- Adult Vaccine Access Coalition - Member.[261]
- Alliance for a Stronger FDA - Member.[262]
- AMR Industry Alliance - Member.[263]
- BIOTECanada - Member company.[264][265]
- Bipartisan Policy Center - Donor.[266]
- The Business Council - Member, represented by CEO Albert Bourla.[267]
- Business Council for the United Nations - Member.[268]
- Center on Budget and Policy Priorities - Funder.[269]
- Community Anti-Drug Coalitions of America (CADCA) - Partner.[270]
- COVID-19 Vaccine Education and Equity Project - Sponsor.[271]
- European Federation of Pharmaceutical Industries and Associations - Member.[272]
- Foundation for the National Institutes of Health (FNIH) - Donor. Pfizer has given between $5,000,000 and $9,999,999 to the between 1997 and 2020, contributing to funding the activities of the National Institutes of Health.[273]
- Global Health Council - Member.[274]
- Immunisation Coalition (Australia) - Sponsor.[275]
- Innovative Medicines Canada - Member. IMC is an association of pharmaceutical companies doing business in Canada.[276][277] The group lobbies the Government of Ontario and House of Commons of Canada through Rubicon Strategy, a firm owned by Progressive Conservative Party of Ontario campaign manager Kory Teneycke.[278][279][280]
- International Federation of Pharmaceutical Manufacturers & Associations (IFPMA) - Member.[281]
- Life Sciences British Columbia (LSBC) - Member company and Platinum Sponsor.[282][283]
- National Health Council (NHC) - Member organization. NHC is a non-profit organization that lobbies the U.S. Government on issues related to healthcare reform.[284]
- National Pharmaceutical Council (NPC) - Member company.[285]
- Personalized Medicine Coalition (PMC) - Member.[286][287]
- Pharmaceutical Advertising Advisory Board (PAAB) - Client.[288]
- Pharmaceutical Research and Manufacturers of America (PhRMA) - Member company.[289][290]
- Reagan-Udall Foundation for the Food and Drug Administration - Donor.[291]
- Research!America - Member organization.[292]
- U.S. Global Leadership Coalition - Member.[293]
- World Economic Forum - Member organization.[294][295]
Scott Gottlieb, who resigned as FDA commissioner in April 2019, joined the Pfizer board of directors three months later, in July 2019.[296]
Pfizer lobbied various officials in the Government of British Columbia between April and November 2012, including then-premier Christy Clark, future premier John Horgan, future health minister Adrian Dix, and future deputy premier, minister of public safety and solicitor general Mike Farnworth. The disclosed purpose was to "provide health policy and pharmaceutical information and communications on behalf of Pfizer Canada," and "learn and understand the budgetary, policy and strategic directions of the Government."[297]
Professional associations
- Academy of Surgical Research (ASR) - 2021 Annual Meeting sponsor.[298]
- American Statistical Association (ASA) - Corporate supporter.[299]
- Bioscience Association Manitoba (BAM) - Sponsor.[300]
- British Columbia Pharmacy Association (BCPA) - Event sponsor.[301]
- Canadian Association for Clinical Microbiology and Infectious Diseases (CACMID) - Patron (former).[302]
- Canadian Association of Emergency Physicians (CAEP) - Corporate partner.[303]
- Canadian Association of Medical Oncologists - Annual meeting sponsor.[304]
- Canadian Medical Association - Sponsor. In 2009, Pfizer partnered with the CMA to launch a continuing medical education course for physicians.[305]
- Canadian Pharmacists Association and Canadian Pharmacists Journal - Sponsor.[306]
- Canadian Public Health Association - Sponsor.[307]
- Canadian Rheumatology Association - Sponsor.[308]
- Canadian Urological Association - Sponsor.[309][310]
- Ontario Medical Association (OMA) - Donor to the Ontario Medical Foundation.[311]
- Pharmacy Association of Nova Scotia - Sponsor.[312]
Public health
Pfizer has engaged in a number of public health and global health initiatives worldwide, and provides funding for health care facilities of various specialties in Canada and the United States:
- CANImmunize - Endorsing partner.[149] CANImmunize is a vaccine passport software company funded primarily by the Public Health Agency of Canada, and partnered with governments, health agencies, academia and pharmaceutical companies across Canada.
- Centre for Addiction and Mental Health - Donor.[313]
- Dana–Farber Cancer Institute - Donor.[314]
- Federation of Medical Women of Canada - Sponsor.[315][316]
- Food Allergy Canada - Corporate partner, providing funding and advocacy support.[317][318]
- Hospital for Sick Children (SickKids) - Donor to the SickKids Foundation.[319]
- Medical Teams International - Corporate donor.[320]
- North Bay Regional Health Center - Donor to the NBRHC Foundation.[321]
- Princess Margaret Cancer Centre (PMCC) - Conference sponsor,[322] and donor to the Princess Margaret Cancer Foundation.[323]
- Scarborough Health Network (SHN) - Donor to the SHN Foundation.[324]
- Sinai Health Foundation - Donor. The foundation funds Mount Sinai Hospital, Bridgepoint Active Healthcare, and the Lunenfeld-Tanenbaum Research Institute in Toronto, Ontario.[325]
- Sunnybrook Health Sciences Centre - Donor.[326]
- University Hospitals Kingston Foundation - Donor.[327] UHKF raises funds for the Kingston Health Sciences Centre and Providence Care.
- William Osler Health System - Event sponsor.[328]
Pfizer sponsored a presentation in January 2020 delivered by Julie Bettinger through British Columbia's Provincial Health Services Authority (PHSA) titled "Vaccine hesitancy: It doesn't matter if the vaccine works if nobody gets it."[256][329]
In 2020, Pfizer provided funding in the range of $100,000.00 - $250,000.00 to Ronald McDonald House Charities “to provide resources that directly improve the health and well-being of children and their families.”[330]
Research and development
Pfizer has partnered with and sponsored many medical research networks and professional associations in the United States, Canada and globally:
- ABC Global Alliance - Main sponsor.[331] The alliance is a Portuguese not-for-profit society supporting research into advanced breast cancer.
- Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) - Industry partner.
- AdvaMed - Member (former).[332]
- Alliance for Regenerative Medicine - Member organization.[333] The alliance is an international advocacy organization supporting the development of regenerative medicines including gene therapy and stem-cell therapy.
- Arthritis Australia - Donor.[334]
- BioFIT - Sponsor.[335] BioFIT holds events to connect academia, pharmaceutical companies, and investors in the field of life sciences and biotechnology.
- Canadian Frailty Network - Industry partner.[336] CFN has provided research grants related to COVID-19.[337]
- Colorectal Cancer Canada - Sponsor.[338][339]
- Drugs for Neglected Diseases Initiative - Partner.[340] DNDI is a non-profit drug research and development organization that expedites creation and delivery of medicines for diseases including leishmaniasis, sleeping sickness, and hepatitis C.
- GISAID - Funding for COVID-19 operations.[341]
- Heart and Stroke Foundation of Canada - National corporate partner and sponsor.[342][343]
- Lung Health Foundation - Partner.[344] Funds research into infectious lung disease and lobbying for policy changes.
- Mentoring in IBD - Sponsor. Annual educational program for Canadian gastroenterologists.[345]
- Mount Sinai Hospital (Toronto) - Sponsor for research into infectious diseases such as COVID-19 through educational grants.[346]
- Nova Scotia Chronic Pain Collaborative Care Network - Investment in Canadian health research.[347]
- Ontario Hospital Research Institute (OHRI) - Research grants.[348]
- Pinnacle Research Group - Sponsor.[349]
- Radcliffe Cardiology - Industry partner.[350]
- Truth Initiative - Featured partner.[351] The initiative performs research and policy studies related to the reduction of tobacco use in youth.
See also
- Biotech and pharmaceutical companies in the New York metropolitan area
- Companies of the United States with untaxed profits
- Fire in the Blood (2013 film)
- List of pharmaceutical companies
Purdue pharma
References
- ↑ 1.0 1.1 1.2 1.3 "Pfizer Inc. 2022 Form 10-K Annual Report". U.S. Securities and Exchange Commission. February 23, 2023. https://www.sec.gov/ix?doc=/Archives/edgar/data/78003/000007800323000024/pfe-20221231.htm.
- ↑ Wells, John C. (2008), Longman Pronunciation Dictionary (3rd ed.), Longman, ISBN 9781405881180
- ↑ Bomey, Nathan (August 25, 2020). "Exxon Mobil, Pfizer removed from Dow Jones Industrial Average; Salesforce, Honeywell added". USA Today. https://www.usatoday.com/story/money/2020/08/25/exxon-pfizer-dow-jones-industrial-average-salesforce-djia/5630916002/.
- ↑ Stevens, Pippa (August 24, 2020). "Salesforce, Amgen and Honeywell added to Dow in major shake-up to the average". CNBC. https://www.cnbc.com/2020/08/24/salesforce-amgen-and-honeywell-added-to-dow-in-major-shakeup-to-the-average.html.
- ↑ Ponczek, Sarah; Greifeld, Katherine (August 24, 2020). "Exxon Booted from Dow Industrials in Major Embrace of Tech". Bloomberg News. https://www.bloomberg.com/news/articles/2020-08-24/dow-industrials-kicks-out-exxon-in-biggest-shakeup-since-2013.
- ↑ Levisohn, Ben (August 25, 2020). "Exxon and Pfizer Just Got Booted From the Dow. Here's What's Replacing Them.". Barron's. https://www.barrons.com/articles/exxon-and-pfizer-just-got-booted-from-the-dow-jones-industrial-average-heres-whats-replacing-them-51598307204.
- ↑ "Fortune 500: Pfizer". Fortune. https://fortune.com/company/pfizer/fortune500/.
- ↑ "Forbes Global 2000: Pfizer". Forbes. https://www.forbes.com/companies/pfizer/.
- ↑ "Pfizer Inc.". International Directory of Company Histories. 178. Internet Archive. St. James Press. 2016. pp. 362–373. ISBN 978-1-4103-9198-8. http://archive.org/details/internationaldir0178unse.
- ↑ 10.0 10.1 Kenneth T. Jackson. The Encyclopedia of New York City. The New York Historical Society; Yale University Press; September 1995. P. 895. ISBN:978-0-300-05536-8
- ↑ "Pfizer's Birthplace, Soon Without Pfizer". The New York Times. January 28, 2007. https://www.nytimes.com/2007/01/28/nyregion/28pfizer.html.
- ↑ 12.0 12.1 "Guide to the Pfizer Inc. collection ARC.084". Brooklyn Public Library. http://dlib.nyu.edu/findingaids/html/bhs/arc_084_pfizer/bioghist.html.
- ↑ 13.0 13.1 13.2 13.3 "Company Timeline: a Legacy of Innovation". https://www.pfizer.com/about/history.
- ↑ 14.0 14.1 "Penicillin Production through Deep-tank Fermentation – National Historic Chemical Landmark". American Chemical Society. https://www.acs.org/content/acs/en/education/whatischemistry/landmarks/penicillin.html.
- ↑ Johnson, Steven (2021) (in en). Extra Life (1st ed.). Riverhead Books. pp. 160. ISBN 978-0-525-53885-1.
- ↑ "Fluconazole". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/fluconazole.html.
- ↑ "Azithromycin: A world best-selling Antibiotic". World Intellectual Property Organization. https://www.wipo.int/ipadvantage/en/details.jsp?id=906.
- ↑ "Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults". Clinical Infectious Diseases 44 (Suppl 2): S27–72. March 2007. doi:10.1086/511159. PMID 17278083. PMC 7107997. https://www.thoracic.org/statements/resources/mtpi/idsaats-cap.pdf.
- ↑ Wilson, Jacque (March 27, 2013). "Viagra: The little blue pill that could". CNN. https://www.cnn.com/2013/03/27/health/viagra-anniversary-timeline/index.html.
- ↑ Cox, David (June 9, 2019). "The race to replace Viagra". The Guardian. https://www.theguardian.com/science/2019/jun/09/race-to-replace-viagra-patents-erectile-dysfunction-drug-medical-research-cialis-eroxon.
- ↑ "Pfizer Inc., New York, has elected its...". Los Angeles Times. March 29, 1991. https://www.latimes.com/archives/la-xpm-1991-03-29-fi-903-story.html.
- ↑ "Kenneth Koe '45" (in en-us). Reed College. December 2015. https://www.reed.edu/reed-magazine/in-memoriam/obituaries/_online_only/kenneth-koe-1945.html.
- ↑ Smith, Aaron (2006-04-04). "Who stands to gain when Zoloft goes generic?". CNN Money. https://money.cnn.com/2006/04/04/news/companies/antidepressants/.
- ↑ "Highlights of Prescribing Information". Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020690s035,021720s008,022568s005lbl.pdf.
- ↑ "Drug Approval Package". Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/nda/96/19-787s007_Amlodipine.cfm.
- ↑ Mehta, Praful (2011-11-29). "Lipitor Patent Expiration - The End of an Era for Atorvastatin Sales". IHS Markit. https://ihsmarkit.com/research-analysis/lipitor-patent-expiration-atorvastatin-sales.html.
- ↑ 27.0 27.1 Berenson, Alex (July 29, 2006). "A Long Shot Becomes Pfizer's Latest Chief Executive". The New York Times. https://www.nytimes.com/2006/07/29/business/29pfizer.html.
- ↑ Bank, David; Buckman, Rebecca (2002-05-17). "Gates Foundation Buys Stakes in Drug Makers" (in en-US). The Wall Street Journal. ISSN 0099-9660. https://www.wsj.com/articles/SB1021577629748680000.
- ↑ "Pregabalin". American Society of Health-System Pharmacists. https://www.drugs.com/monograph/pregabalin.html.
- ↑ Frampton, James E. (September 2014). "Pregabalin: A Review of its Use in Adults with Generalized Anxiety Disorder". CNS Drugs 28 (9): 835–854. doi:10.1007/s40263-014-0192-0. PMID 25149863.
- ↑ Iftikhar, I. H.; Alghothani, L.; Trotti, L. M. (December 2017). "Gabapentin enacarbil, pregabalin and rotigotine are equally effective in restless legs syndrome: a comparative meta-analysis". European Journal of Neurology 24 (12): 1446–1456. doi:10.1111/ene.13449. PMID 28888061.
- ↑ Decker, Susan (February 6, 2014). "Pfizer Wins Ruling to Block Generic Lyrica Until 2018". Bloomberg News. https://www.bloomberg.com/news/2014-02-06/pfizer-wins-ruling-to-block-generic-lyrica-until-2018.html.
- ↑ "Pfizer names new CEO". CNN. July 28, 2006. https://money.cnn.com/2006/07/28/news/companies/pfizer_ceo/index.htm.
- ↑ Berenson, Alex; Pollack, Andrew (December 5, 2006). "Pfizer Shares Plummet on Loss of a Promising Heart Drug". The New York Times. https://www.nytimes.com/2006/12/05/health/05pfizer.html.
- ↑ Berenson, Alex (December 3, 2006). "Pfizer Ends Studies on Drug for Heart Disease". The New York Times. https://www.nytimes.com/2006/12/03/health/03pfizer.html.
- ↑ Agovino, Theresa (December 3, 2006). "Pfizer ends cholesterol drug development". Associated Press. The Seattle Times. https://www.seattletimes.com/nation-world/cholesterol-drug-trials-are-halted/.
- ↑ Tanne, Janice Hopkins (16 December 2006). "Pfizer stops clinical trials of heart drug". BMJ 333 (7581): 1237.2–1237. doi:10.1136/bmj.39059.438044.DB. PMID 17170401.
- ↑ Bennett, Simeon (July 8, 2010). "Pfizer: Civil Suits for Drug Counterfeiters". Bloomberg Businessweek. https://www.bloomberg.com/news/articles/2010-07-08/pfizer-civil-suits-for-drug-counterfeiters.
- ↑ Jones, Al (July 15, 2008). "Pfizer job cuts don't equal a reduction in work load, says company spokesman". Booth Newspapers. https://www.mlive.com/kzgazette/2008/07/pfizer_job_cuts_dont_equal_a_r.html.
- ↑ 40.0 40.1 "It's official: Pfizer buys Pharmacia". CNN. April 16, 2003. https://money.cnn.com/2003/04/16/news/companies/pfizer_pharma/.
- ↑ Hensley, Scott (June 20, 2000). "Pfizer Completes Stormy Takeover Of Warner-Lambert for $116 Billion". The Wall Street Journal. https://www.wsj.com/articles/SB961456765639278103.
- ↑ Campbell, Todd (May 15, 2017). "Here are the 7 biggest mergers of all time". The Motley Fool. Business Insider. https://www.businessinsider.com/here-are-the-7-biggest-mergers-of-all-time-2017-5#6-warner-lambert-warms-up-to-pfizer-89-billion-2.
- ↑ "Metronidazole Monograph for Professionals". Drugs.com. https://www.drugs.com/monograph/metronidazole.html.
- ↑ "Celecoxib Monograph for Professionals". American Society of Health-System Pharmacists. 11 November 2019. https://www.drugs.com/monograph/celecoxib.html.
- ↑ "The Spirit Of A Startup Lives On". Bloomberg Businessweek. November 21, 2005. https://www.bloomberg.com/news/articles/2005-11-20/the-spirit-of-a-startup-lives-on.
- ↑ "Pfizer expects to shutter South City biotech outpost". American City Business Journals. April 30, 2003. https://www.bizjournals.com/sanfrancisco/stories/2003/04/28/daily24.html.
- ↑ Rockoff, Jonathan D. (August 26, 2011). "FDA Approves Pfizer Lung-Cancer Drug". The Wall Street Journal. https://www.wsj.com/articles/SB10001424053111904009304576532892704206326.
- ↑ Mortlock, A.A.; Wilson, D.M.; Kettle, J.G.; Goldberg, F.W.; Foote, K.M. (2017). "Selective Kinase Inhibitors in Cancer". Comprehensive Medicinal Chemistry III. pp. 39–75. doi:10.1016/B978-0-12-409547-2.12391-1. ISBN 978-0-12-803201-5.
- ↑ Barriaux, Marianne (October 9, 2006). "Pfizer buys vaccine developer PowderMed". The Guardian. https://www.theguardian.com/business/2006/oct/09/money5.
- ↑ Sorkin, Andrew Ross; Wilson, Duff (January 25, 2009). "Pfizer Agrees to Pay $68 Billion for Rival Drug Maker Wyeth". The New York Times. ISSN 0362-4331. https://www.nytimes.com/2009/01/26/business/26drug.html.
- ↑ "Pfizer completes $67 billion deal for rival Wyeth". Reuters. October 15, 2009. https://www.reuters.com/article/instant-article/idUSTRE59E4S320091015.
- ↑ Karnitschnig, Matthew; Rockoff, Jonathan D. (January 23, 2009). "Pfizer in Talks to Buy Wyeth". The Wall Street Journal. https://www.wsj.com/articles/SB123268511212809429.
- ↑ Edwards, Jim (January 23, 2009). "The Pfizer–Wyeth Deal Worst-Case Scenario". CBS News. https://www.cbsnews.com/news/the-pfizer-wyeth-deal-worst-case-scenario/.
- ↑ 54.0 54.1 "PFIZER COMPLETES ACQUISITION OF WYETH" (Press release). Pfizer. October 14, 2009.
- ↑ "CDC – ABCs: Surveillance Reports main page – Active Bacterial Core surveillance". April 5, 2019. https://www.cdc.gov/abcs/reports-findings/surv-reports.html.
- ↑ Herper, Matthew (August 24, 2020). "In the race for a Covid-19 vaccine, Pfizer turns to a scientist with a history of defying skeptics – and getting results". https://www.statnews.com/2020/08/24/pfizer-edge-in-the-race-for-a-covid-19-vaccine-could-be-a-scientist-with-two-best-sellers-to-her-credit/.
- ↑ 57.0 57.1 Liu, Angus; Kansteiner, Fraiser (April 4, 2023). "Pfizer moves on up to the West Side, establishing new nerve center at Hudson Yards' Spiral skyscraper". Fierce Pharma. https://www.fiercepharma.com/pharma/pfizer-moves-west-side-settling-down-swanky-new-digs-hudson-yards-spiral-skyscraper. Retrieved April 6, 2023.
- ↑ "Ian Read to Retire as Executive Chairman of Pfizer's Board of Directors; Chief Executive Officer Dr. Albert Bourla Named Chairman" (Press release). Business Wire. September 27, 2019. External link in
|publisher=(help) - ↑ "Pfizer to close UK research site". BBC News. February 1, 2011. https://www.bbc.co.uk/news/business-12335801.
- ↑ Mckinney, Maureen (March 1, 2011). "Pfizer closes King Pharmaceuticals acquisition". Modern Healthcare. https://www.modernhealthcare.com/article/20110301/NEWS/303019922/pfizer-closes-king-pharmaceuticals-acquisition.
- ↑ Yukhananov, Anna (September 4, 2012). "FDA approves Pfizer leukemia drug". Reuters. https://www.reuters.com/article/us-pfizer-leukemia/fda-approves-pfizer-leukemia-drug-idUSBRE88314720120904.
- ↑ "Drug Approval Package". Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203214orig1s000toc.cfm.
- ↑ Sagonowsky, Eric (February 20, 2019). "Pfizer switches RA patients to lower dose of fast-growing Xeljanz as safety issues arise in postmarketing study". Fierce Pharma. https://www.fiercepharma.com/pharma/postmarketing-study-pfizer-switches-ra-patients-to-lower-xeljanz-dose-safety-concerns.
- ↑ "Zoetis™ Files IPO Registration Statement" (Press release). Business Wire. August 13, 2012. External link in
|publisher=(help) - ↑ J. de la Merced, Michael (February 1, 2013). "Shares of Zoetis Surge on Debut". The New York Times. https://dealbook.nytimes.com/2013/02/01/shares-of-zoetis-surge-on-debut/.
- ↑ Dieterich, Chris (January 31, 2013). "Zoetis Raises $2.2 Billion in IPO". The Wall Street Journal. https://www.wsj.com/articles/SB10001424127887323701904578276530830057770.
- ↑ "Shares of animal health company Zoetis soar in IPO". Associated Press. CBS News. February 1, 2013. https://www.cbsnews.com/news/shares-of-animal-health-company-zoetis-soar-in-ipo/.
- ↑ Loftus, Peter (May 22, 2013). "Pfizer to Spin Off Remaining Zoetis Stake". The Wall Street Journal. https://www.wsj.com/articles/SB10001424127887323975004578498994013821124.
- ↑ Humer, Caroline; Pierson, Ransdell (May 22, 2013). "Pfizer to spin off Zoetis stake to shareholders". Reuters. https://www.reuters.com/article/us-pfizer-zoetis/pfizer-to-spin-off-zoetis-stake-to-shareholders-idUSBRE94L0JB20130522.
- ↑ Wasserman, Emily (September 29, 2014). "Pfizer Completes Acquisition Of InnoPharma". Fierce Pharma. https://www.fiercepharma.com/pharma/pfizer-completes-acquisition-of-innopharma.
- ↑ "Pfizer to Acquire InnoPharma for Up to $360M". July 16, 2014. https://www.genengnews.com/news/pfizer-to-acquire-innopharma-for-up-to-360m/.
- ↑ "Pfizer Buys Redvax, Boosting Vaccine Portfolio". January 5, 2015. https://www.genengnews.com/topics/drug-discovery/pfizer-buys-redvax-boosting-vaccine-portfolio/.
- ↑ Beaver, Julia A.; Amiri-Kordestani, Laleh; Charlab, Rosane; Chen, Wei; Palmby, Todd; Tilley, Amy; Zirkelbach, Jeanne Fourie; Yu, Jingyu et al. (1 November 2015). "FDA Approval: Palbociclib for the Treatment of Postmenopausal Patients with Estrogen Receptor–Positive, HER2-Negative Metastatic Breast Cancer". Clinical Cancer Research 21 (21): 4760–4766. doi:10.1158/1078-0432.CCR-15-1185. PMID 26324739.
- ↑ "Palbociclib (IBRANCE)". Food and Drug Administration. February 9, 2019. https://www.fda.gov/drugs/resources-information-approved-drugs/palbociclib-ibrance.
- ↑ "Pfizer, Lilly to Resume Phase III Tanezumab Clinical Program". March 23, 2015. https://www.genengnews.com/topics/drug-discovery/pfizer-lilly-to-resume-phase-iii-tanezumab-clinical-program/.
- ↑ Gali, Weinreb (May 14, 2015). "Pfizer to collaborate on Bar-Ilan DNA robots". Globes. https://en.globes.co.il/en/article-pfizer-to-collaborate-on-bar-ilan-dna-robots-1001036703.
- ↑ "Pfizer Buys Two GSK Meningitis Vaccines for $130M". June 22, 2015. https://www.genengnews.com/topics/drug-discovery/pfizer-buys-two-gsk-meningitis-vaccines-for-130m/.
- ↑ "Pfizer Completes Acquisition of Hospira" (Press release). Pfizer. September 3, 2015 – via Business Wire.
- ↑ "Pfizer completes $17-billion Hospira acquisition". The Pharma Letter. September 4, 2015. https://www.thepharmaletter.com/article/pfizer-completes-17-billion-hospira-acquisition.
- ↑ Gelles, David; Thomas, Katie (February 5, 2015). "Pfizer Bets $15 Billion on New Class of Generic Drugs". The New York Times. https://dealbook.nytimes.com/2015/02/05/pfizer-to-buy-hospira-a-drug-maker-for-15-2-billion-in-cash.
- ↑ "8-K". U.S. Securities and Exchange Commission. February 6, 2015. https://www.sec.gov/Archives/edgar/data/78003/000119312515037588/d866443d8k.htm.
- ↑ "Pfizer to Acquire Hospira". Pfizer (Press release).
- ↑ Neilan, Catherine (February 5, 2015). "Pfizer, Hospira share prices to soar after $17bn deal announced". City A.M.. https://www.cityam.com/pfizer-hospira-share-prices-soar-after-17bn-deal-announced/.
- ↑ Bhalla, Mohit; Singh, Khomba (December 16, 2009). "US-based Hospira to buy Orchid Chemicals' injectables biz for $400 mn". The Economic Times. https://m.economictimes.com/industry/healthcare/biotech/pharmaceuticals/us-based-hospira-to-buy-orchid-chemicals-injectables-biz-for-400-mn/articleshow/5342003.cms.
- ↑ "Pfizer seals $160bn Allergan deal to create drugs giant". BBC News. November 23, 2015. https://www.bbc.co.uk/news/business-34900344.
- ↑ Pierson, Ransdell; Berkrot, Bill (November 24, 2015). "Pfizer to buy Allergan in $160 billion deal". Reuters. https://www.reuters.com/article/us-allergan-m-a-pfizer/pfizer-to-buy-allergan-in-160-billion-deal-idUSKBN0TB0UT20151124.
- ↑ "Pfizer to Acquire Allergan for $160B". genengnews.com. November 23, 2015. https://www.genengnews.com/news/pfizer-to-acquire-allergan-for-160b/.
- ↑ Koons, Cynthia (November 22, 2015). "Pfizer and Allergan to Combine With Joint Value of $160 Billion". Bloomberg News. https://www.bloomberg.com/news/articles/2015-11-22/pfizer-allergan-said-to-be-close-to-150-billion-merger.
- ↑ Bray, Chad (April 6, 2016). "Pfizer and Allergan Call Off Merger After Tax-Rule Changes". The New York Times. https://www.nytimes.com/2016/04/07/business/dealbook/pfizer-allergan-merger.html.
- ↑ Humer, Caroline; Banerjee, Ankur (April 6, 2016). "Pfizer, Allergan scrap $160 billion deal after U.S. tax rule change". Reuters. https://www.reuters.com/article/us-allergan-m-a-pfizer-idUSKCN0X3188.
- ↑ "Pfizer Completes Acquisition of Anacor" (Press release). Pfizer. June 24, 2016 – via Business Wire.
- ↑ "Pfizer to Acquire Anacor Pharmaceuticals for $5.2B". May 16, 2016. https://www.genengnews.com/news/pfizer-to-acquire-anacor-pharmaceuticals-for-5-2b/.
- ↑ "Pfizer Places High Bid of $40M for BIND Therapeutics". July 27, 2016. https://www.genengnews.com/news/pfizer-places-high-bid-of-40m-for-bind-therapeutics/.
- ↑ "Pfizer Acquires Bamboo Therapeutics in a $645M Deal". August 1, 2016. https://www.genengnews.com/news/pfizer-acquires-bamboo-therapeutics-in-a-645m-deal/.
- ↑ "Pfizer to Acquire Medivation for $14B". August 22, 2016. https://www.genengnews.com/news/pfizer-to-acquire-medivation-for-14b/.
- ↑ "Pfizer to buy cancer drug firm Medivation for $14bn". BBC News. August 22, 2016. https://www.bbc.co.uk/news/business-37150531.
- ↑ "Pfizer Completes Acquisition of Medivation" (Press release). OncoImmune. September 28, 2016 – via Business Wire.
- ↑ "OncoImmune Licenses ONC-392 to Pfizer for Up to $250M". genengnews.com. October 15, 2016. https://www.genengnews.com/topics/drug-discovery/oncoimmune-licenses-onc-392-to-pfizer-for-up-to-250m/.
- ↑ "OncoImmune Announces Option and License Agreement with Pfizer Inc" (Press release). Pfizer. September 15, 2016 – via Business Wire.
- ↑ 100.0 100.1 100.2 "CDC Foundation Active Programs October 1, 2020 – September 30, 2021". 2021-12-09. https://www.cdcfoundation.org/CDCF-ActivePrograms-CDC-FY21?inline.
- ↑ "Pfizer Completes Acquisition of Small Molecule Anti-Infective Business From AstraZeneca" (Press release). Pfizer. December 23, 2016 – via Business Wire.
- ↑ "Pfizer Buys AstraZeneca Antibiotics for Up to $1.575B". August 24, 2016. https://www.genengnews.com/topics/drug-discovery/pfizer-buys-astrazeneca-antibiotics-for-up-to-1-575b/.
- ↑ Staton, Tracy (August 24, 2016). "Pfizer grabs AZ antibiotics in $1.5B deal. Pre-split prep or just another sales-boosting buy?". Fierce Pharma. https://www.fiercepharma.com/pharma/pfizer-grabs-az-antibiotics-1-5b-deal-pre-split-prep-or-just-another-sales-boosting-buy.
- ↑ Hiltzik, Michael (January 8, 2018). "Pfizer, pocketing a big tax cut from Trump, will end investment in Alzheimer's and Parkinson's research". Los Angeles Times. https://www.latimes.com/business/hiltzik/la-fi-hiltzik-pfizer-20180108-story.html.
- ↑ "FDA approves enzalutamide for castration-resistant prostate cancer" (Press release). Food and Drug Administration. July 13, 2018.
- ↑ "BioNTech Signs Collaboration Agreement with Pfizer to Develop mRNA-based Vaccines for Prevention of Influenza" (Press release). BioNTech. August 16, 2018.
- ↑ Mathias, Tamara; Banerjee, Ankur (October 1, 2018). "Pfizer to replace longtime CEO Read with veteran Bourla". Reuters. https://www.reuters.com/article/us-pfizer-ceo/pfizer-to-replace-longtime-ceo-read-with-veteran-bourla-idUSKCN1MB29D.
- ↑ Maidenberg, Micah (October 9, 2018). "Pfizer Prepares for CEO Transition With Executive Suite Changes". The Wall Street Journal. https://www.wsj.com/articles/pfizer-prepares-for-ceo-transition-with-executive-suite-changes-1539095075.
- ↑ Ramsey, Lydia (October 1, 2018). "Pfizer's CEO is stepping down after 8 years — meet the man who will be replacing him". Business Insider. https://www.businessinsider.com/who-is-albert-bourla-next-pfizer-ceo-2018-10.
- ↑ Jarvis, Lisa M. (October 3, 2018). "Pfizer unveils CEO succession plan". Chemical & Engineering News. https://cen.acs.org/pharmaceuticals/Pfizer-unveils-CEO-succession-plan/96/i40.
- ↑ Bakolia, Ravikash (July 1, 2019). "Pfizer completes acquisition of Therachon to bolster rare disease drug portfolio". S&P Global. https://www.spglobal.com/marketintelligence/en/news-insights/trending/sWCcSrMgL8UmeCJXuuGp-w2.
- ↑ "Pfizer Completes Acquisition of Array Biopharma" (Press release). Pfizer. July 30, 2019 – via Business Wire.
- ↑ "GlaxoSmithKline and Pfizer merge healthcare arms". BBC News. December 19, 2018. https://www.bbc.co.uk/news/business-46616713.
- ↑ Helfand, Carly (October 16, 2017). "Reckitt Benckiser's still keen on a Pfizer OTC buy. But can it afford one?". Fierce Pharma. https://www.fiercepharma.com/pharma/reckitt-benckiser-s-still-keen-a-pfizer-otc-buy-but-can-it-afford-one.
- ↑ Helfand, Carly (October 26, 2017). "Sanofi, J&J could join GlaxoSmithKline, Reckitt in $20B bidding war for Pfizer OTC: report". Fierce Pharma. https://www.fiercepharma.com/pharma/sanofi-j-j-could-join-gsk-reckitt-20b-bidding-war-for-pfizer-otc-report.
- ↑ Helfand, Carly (October 25, 2017). "GlaxoSmithKline eyes Pfizer's OTC unit. But will a buy imperil its dividend?". Fierce Pharma. https://www.fiercepharma.com/pharma/glaxosmithkline-s-eyeing-pfizer-s-otc-unit-but-will-a-buy-imperil-its-dividend.
- ↑ "Pfizer to buy 9.9% of CStone for $200 million, eyes collaboration". Reuters. September 29, 2020. https://www.reuters.com/article/us-cstone-pfizer/pfizer-to-buy-9-9-of-cstone-for-200-million-eyes-collaboration-idUKKBN26L01D.
- ↑ Al Idrus, Amirah (October 22, 2020). "Biotech Pfizer snaps up antibiotics maker Arixa and its oral Avycaz follow-up". Fierce Pharma. https://www.fiercebiotech.com/biotech/pfizer-snaps-up-antibiotics-maker-arixa-and-its-oral-avycaz-follow-up.
- ↑ "Pfizer Completes Combination Of Upjohn And Mylan; Viatris To Begin Trading On Nov. 17". Nasdaq. November 16, 2020. https://www.nasdaq.com/articles/pfizer-completes-combination-of-upjohn-and-mylan-viatris-to-begin-trading-on-nov.-17-2020.
- ↑ Sabatini, Patricia (November 16, 2020). "Mylan completes merger with Upjohn to form Viatris". Pittsburgh Post-Gazette. https://www.post-gazette.com/business/healthcare-business/2020/11/16/Mylan-merger-Upjohn-Viatris-bresch-coury-Pfizer-cost-cuts-generic-drugs/stories/202011160111.
- ↑ Bruell, Alexandra (January 5, 2021). "Pfizer Introduces New Logo Playing Up Role in Drug Creation". The Wall Street Journal. ISSN 0099-9660. https://www.wsj.com/articles/pfizer-introduces-new-logo-playing-up-role-in-drug-creation-11609844400.
- ↑ Taylor, Nick Paul (April 28, 2021). "Pfizer buys Amplyx to grow infectious disease pipeline". Fierce Pharma. https://www.fiercebiotech.com/biotech/pfizer-buys-amplyx-to-grow-infectious-disease-pipeline.
- ↑ Wosen, Jonathan (April 28, 2021). "Pfizer acquires fungus-fighting San Diego biotech". The San Diego Union-Tribune. https://www.sandiegouniontribune.com/business/biotech/story/2021-04-28/pfizer-acquires-fungus-fighting-san-diego-biotech-amplyx.
- ↑ "Pfizer to buy cancer drug developer Trillium in $2.3 BLN deal". Reuters. August 23, 2021. https://www.reuters.com/business/pfizer-buy-trillium-therapeutics-226-bln-deal-2021-08-23/.
- ↑ "Pfizer to Acquire Trillium Therapeutics Inc". https://www.biospace.com/article/releases/pfizer-to-acquire-trillium-therapeutics-inc-/?s=79.
- ↑ "Pfizer Completes Acquisition of Arena Pharmaceuticals" (Press release). Business Wire. March 11, 2022. External link in
|publisher=(help) - ↑ "Pfizer bets on Arena's promising bowel disease treatment in $6.7 bln deal". Reuters. December 13, 2021. https://www.reuters.com/markets/deals/pfizer-acquire-arena-pharmaceuticals-67-bln-deal-2021-12-13.
- ↑ Walker, Joseph (December 13, 2021). "Pfizer to Acquire Arena Pharmaceuticals in $6.7 Billion Deal". The Wall Street Journal. https://www.wsj.com/articles/pfizer-to-acquire-arena-pharmaceuticals-in-6-7-billion-deal-11639414813.
- ↑ Satija, Bhanvi (April 7, 2022). "Pfizer boosts respiratory drug portfolio with ReViral purchase". Reuters. https://www.reuters.com/business/healthcare-pharmaceuticals/pfizer-buy-rsv-drug-developer-reviral-up-525-mln-2022-04-07/.
- ↑ "Pfizer acquires biopharma firm ReViral for up to $525m" (in en-US). 2022-06-10. https://www.pharmaceutical-technology.com/news/pfizer-acquires-biopharma-reviral/.
- ↑ "Pfizer Completes Acquisition of Biohaven Pharmaceuticals". October 3, 2022. https://www.biospace.com/article/releases/pfizer-completes-acquisition-of-biohaven-pharmaceuticals/?s=110.
- ↑ "Pfizer to Acquire Biohaven Pharmaceuticals" (Press release). May 10, 2022.
- ↑ "Pfizer Drops a Cool $11.6B on Migraine Leader Biohaven". https://www.biospace.com/article/pfizer-drops-a-cool-11-6-billion-on-migraine-leader-biohaven-/?s=79.
- ↑ "Pfizer Completes Acquisition of Global Blood Therapeutics" (Press release). Business Wire. October 5, 2022. External link in
|publisher=(help) - ↑ Rockoff, Jonathan D. (2022-08-08). "Pfizer Agrees to $5.4 Billion Deal for Global Blood Therapeutics" (in en-US). The Wall Street Journal. ISSN 0099-9660. https://www.wsj.com/articles/pfizer-reaches-5-4-billion-deal-for-global-blood-therapeutics-11659954601.
- ↑ "Flush with cash, Pfizer buys Global Blood Therapeutics in $5.4 billion deal" (in en). Reuters. 2022-08-08. https://www.reuters.com/business/healthcare-pharmaceuticals/pfizer-buy-global-blood-therapeutics-54-bln-deal-2022-08-08/.
- ↑ Mishra, Manas (2022-03-13). "Pfizer signs $43 bln Seagen deal in cancer drug push". Reuters. https://www.reuters.com/markets/deals/pfizer-buy-seagen-deal-valued-43-billion-2023-03-13/.
- ↑ "Pfizer's $43 Billion Seagen Takeover Faces EU Investigation". 2023-07-15. https://www.bloomberg.com/news/articles/2023-07-15/pfizer-s-43-billion-seagen-merger-faces-eu-investigation.
- ↑ D'Ambrosio, Amanda (April 3, 2023). "Pfizer opens global headquarters in Hudson Yards as empty offices reach pandemic levels". Crain New York Business. https://www.crainsnewyork.com/health-care/pfizer-opens-global-headquarters-hudson-yards-empty-offices-reach-pandemic-levels.
- ↑ Kansteiner, Fraiser (2023-08-28). "Pfizer telegraphs 4th-quarter production restart at North Carolina plant damaged by tornado". https://www.fiercepharma.com/pharma/pfizer-telegraphs-fourth-quarter-production-restart-rocky-mount-plant-damaged-tornado.
- ↑ Staines, Richard (2020-03-26). "Pharma giants including Novartis collaborate on COVID-19 therapies" (in en-GB). https://pharmaphorum.com/news/collaborate-covid19-therapies/.
- ↑ "Advancing research into accessible coronavirus treatments" (in en-US). https://www.therapeuticsaccelerator.org/.
- ↑ "Announcing the COVID-19 Therapeutics Accelerator" (in en). 2020-03-10. https://www.gatesfoundation.org/ideas/articles/coronavirus-mark-suzman-therapeutics.
- ↑ Au-Yeung, Angel (2020-04-03). "A Bill Gates-Backed Accelerator For COVID-19 Therapeutics Treatment Partners With Madonna And Mark Zuckerberg's Chan Zuckerberg Initiative" (in en). https://www.forbes.com/sites/angelauyeung/2020/04/03/a-bill-gates-backed-accelerator-for-covid-19-coronavirus-therapeutics-treatment-partners-with-madonna-and-mark-zuckerbergs-chan-zuckerberg-initiative/.
- ↑ "Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV)" (in EN). https://www.nih.gov/research-training/medical-research-initiatives/activ.
- ↑ University of Minnesota, International Network for Strategic Initiatives in Global HIV Trials (INSIGHT), University of Copenhagen, Medical Research Council, Kirby Institute, Washington D.C. Veterans Affairs Medical Center, AIDS Clinical Trials Group (2022-03-04). A Multicenter, Adaptive, Randomized, Blinded Controlled Trial of the Safety and Efficacy of Investigational Therapeutics for Hospitalized Patients With COVID-19. https://clinicaltrials.gov/ct2/show/NCT04501978.
- ↑ Wholley, David (2020-06-12). "Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV)". https://www.bio.org/sites/default/files/2020-06/ACTIV-BIO-webinar-6-12-20-final.pdf.
- ↑ U.S. Department of State; USAID; Gavi, the Vaccine Alliance (2021-04-15). "The Gavi COVAX AMC Investment Opportunity Launch Event Participant List". https://www.gavi.org/sites/default/files/covid/covax/Investment-Opportunity-Launch-Participant-List.pdf.
- ↑ 149.0 149.1 "Partners". 2022. https://www.canimmunize.ca/en/partners.
- ↑ Czachor, Emily (2020-11-09). "Pfizer Avoided R&D Funding From Trump's Operation Warp Speed Because of Bureaucracy, Politics". Newsweek. https://www.newsweek.com/pfizer-avoided-rd-funding-trumps-operation-warp-speed-because-bureaucracy-politics-1546110.
- ↑ Bianchini, Elisabetta (2020-11-10). "Acuitas Therapeutics: The Canadian technology that the Pfizer and BioNTech COVID-19 vaccine 'can't work without'" (in en-CA). https://ca.news.yahoo.com/covid19-vaccine-coronavirus-canada-acuitas-therapeutics-pfizer-biontech-211410040.html.
- ↑ DeArment, Alaric (2020-07-13). "Pfizer, BioNTech get fast-track from FDA for Covid-19 vaccines". https://medcitynews.com/2020/07/pfizer-biontech-get-fast-track-from-fda-for-covid-19-vaccines/.
- ↑ "Pfizer Beats Forecasts as Vaccine Trial Enters Final Stage". July 29, 2020. https://www.wsj.com/articles/pfizer-sales-fall-as-company-races-toward-covid-19-vaccine-11595936314.
- ↑ Kilgore, Tomi. "Pfizer, BioNTech conclude talks over supplying EU with SARS-CoV-2 vaccine candidate". https://www.marketwatch.com/story/pfizer-biontech-conclude-talks-over-supplying-eu-with-sars-cov-2-vaccine-candidate-2020-09-09.
- ↑ "Covid vaccine: First 'milestone' vaccine offers 90% protection". BBC News. November 9, 2020. https://www.bbc.com/news/health-54873105.
- ↑ 156.0 156.1 Kounang, Nadia (9 November 2020). "Pfizer and BioNTech say final analysis shows coronavirus vaccine is 95% effective with no safety concerns". https://www.cnn.com/2020/11/18/health/pfizer-coronavirus-vaccine-safety/index.html.
- ↑ Thomas, Katie; Gelles, David; Zimmer, Carl (2020-11-09). "Pfizer's Early Data Shows Vaccine Is More Than 90% Effective" (in en-US). The New York Times. ISSN 0362-4331. https://www.nytimes.com/2020/11/09/health/covid-vaccine-pfizer.html.
- ↑ Roberts, Michelle (2 December 2020). "Covid-19: Pfizer/BioNTech vaccine judged safe for use in UK". https://www.bbc.com/news/health-55145696.
- ↑ "Bahrain becomes second country to approve Pfizer COVID-19 vaccine". www.aljazeera.com (Al Jazeera). 4 December 2020. https://www.aljazeera.com/news/2020/12/4/bahrain-becomes-second-country-to-approve-pfizer-covid-19-vaccine.
- ↑ Austen, Ian (9 December 2020). "Canada Approves Vaccine and Could Start Shots Next Week". The New York Times. https://www.nytimes.com/2020/12/09/world/americas/canada-vaccine-approve.html.
- ↑ "Saudi Arabia approves Pfizer COVID-19 vaccine as Bahrain plans to give the public free shots". KTLA. 11 December 2020. https://ktla.com/news/nationworld/saudi-arabia-approves-pfizer-covid-19-vaccine-as-bahrain-plans-to-give-the-public-free-shots/.
- ↑ 162.0 162.1 Steenhuysen, Manas Mishra, Julie (11 December 2020). "U.S. FDA advisers overwhelmingly back authorizing Pfizer–BioNTech COVID-19 vaccine". Reuters. https://www.reuters.com/article/health-coronavirus-pfizer-vaccine/us-fda-advisers-wrestle-with-ethical-issues-linked-to-authorizing-pfizers-covid-19-vaccine-idUSKBN28K1O5.
- ↑ Thomas, Katie; LaFraniere, Sharon; Weiland, Noah; Goodnough, Abby; Haberman, Maggie (2020-12-12). "F.D.A. Clears Pfizer Vaccine, and Millions of Doses Will Be Shipped Right Away". The New York Times. ISSN 0362-4331. https://www.nytimes.com/2020/12/11/health/pfizer-vaccine-authorized.html.
- ↑ Abdullah, Zhaki (December 14, 2020). "Pfizer–BioNTech COVID-19 vaccine approved by Singapore, first shipment expected by end-December". https://www.channelnewsasia.com/news/singapore/singapore-approves-pfizer-biontech-covid-19-vaccine-phase-3-13769570.
- ↑ "EMA recommends first COVID-19 vaccine for authorisation in the EU". December 21, 2020. https://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-authorisation-eu.
- ↑ Higgins-Dunn, Noah (2021-05-04). "BioNTech, with partner Pfizer, on track to make 3B COVID vaccine doses in 2021, CEO says". Fierce Pharma. https://www.fiercepharma.com/pharma/thanks-to-revved-up-manufacturing-biontech-ceo-estimates-3b-covid-vaccine-doses-2021. Retrieved 2021-05-18.
- ↑ "Package Insert - Comirnaty". December 2021. https://www.fda.gov/media/151707/download.
- ↑ Evans, Simon (2021-02-15). "Ansell doubles production as extraordinary demand to stay" (in en). https://www.afr.com/companies/manufacturing/ansell-doubles-production-as-extraordinary-demand-to-stay-20210215-p572p0.
- ↑ 169.0 169.1 "Investigation: Drugmaker 'bullied' Latin American nations". March 11, 2021. https://www.aljazeera.com/news/2021/3/11/investigation-pfizer-bullied-latin-american-nations.
- ↑ Thacker, Paul. "Covid-19: Researcher blows the whistle on data integrity issues in Pfizer's vaccine trial". thebmj. https://www.bmj.com/content/375/bmj.n2635.
- ↑ European Medicines Agency (3 December 2021). "Reply letter on the Integrity of clinical data, additional clinical trials and studies, pharmacovigilance and mRNA COVID-19 vaccine safety". https://www.ema.europa.eu/en/documents/other/ema-reply-letter-members-european-parliament-ms-m-rivasi-mr-p-pedicini-ms-t-metz-concerning-covid-19_en.pdf.
- ↑ Gorski, David (November 8, 2021). "What the heck happened to The BMJ?". Science-Based Medicine. https://sciencebasedmedicine.org/what-the-heck-happened-to-the-bmj/.
- ↑ "Special Committee on COVID-19 pandemic" (in en). 2022-10-10. https://multimedia.europarl.europa.eu/en/webstreaming/special-committee-on-covid-19-pandemic_20221010-1430-COMMITTEE-COVI.
- ↑ TN World Desk (2022-10-13). "Pfizer admits it did not know whether its Covid vaccine prevented transmission of virus when rollout began" (in en). https://www.timesnownews.com/world/pfizer-admits-it-did-not-know-its-covid-vaccine-prevented-transmission-of-virus-when-rollout-began-article-94836651.
- ↑ Chung, Frank (2022-10-13). "Pfizer did not know whether Covid vaccine stopped transmission before rollout, executive admits". https://www.news.com.au/technology/science/human-body/pfizer-did-not-know-whether-covid-vaccine-stopped-transmission-before-rollout-executive-admits/news-story/f307f28f794e173ac017a62784fec414.
- ↑ Bauer-Babef, Clara (2022-10-12). "Pfizer remains discreet about COVID vaccine purchase contracts" (in en-GB). https://www.euractiv.com/section/coronavirus/news/pfizer-remains-discreet-about-covid-vaccine-purchase-contracts/.
- ↑ "Scientific studies show that the Pfizer-BioNTech COVID-19 vaccine reduces transmission; claim by Rob Roos is misleading" (in en-GB). 2022-10-11. https://healthfeedback.org/claimreview/scientific-studies-show-pfizer-biontech-covid-19-vaccine-reduces-transmission-claim-rob-roos-misleading.
- ↑ "Pfizer CEO: Current wave will be last with so many restrictions". https://www.israelnationalnews.com/news/320624.
- ↑ "Pfizer CEO shares some good news on covid but cautions virus could circulate for years". https://www.livemint.com/companies/news/pfizer-ceo-shares-some-good-news-on-covid-but-cautions-virus-could-circulate-for-years-11642405004050.html.
- ↑ Hopkins, Jared S. (2022-05-10). "Some Covid-19 Patients Relapse After Taking Paxlovid, Puzzling Doctors" (in en-US). The Wall Street Journal. ISSN 0099-9660. https://www.wsj.com/articles/some-covid-19-patients-relapse-after-taking-paxlovid-puzzling-doctors-11652186194.
- ↑ Farley, John (2022-05-04). "FDA Updates on Paxlovid for Health Care Providers" (in en). Food and Drug Administration. https://www.fda.gov/drugs/news-events-human-drugs/fda-updates-paxlovid-health-care-providers.
- ↑ Samuels, Fionna M. D. (2022-08-08). "What Is Paxlovid Rebound, and How Common Is It?" (in en). https://www.scientificamerican.com/article/what-is-paxlovid-rebound-and-how-common-is-it/.
- ↑ Kirkpatrick, David D. (May 15, 2000). "Inside the Happiness Business". New York. https://nymag.com/nymetro/health/features/3122/.
- ↑ Oldani, Michael (2002). "Tales from the Script". Kroeber Society Papers 87: 147–176. https://digitalassets.lib.berkeley.edu/anthpubs/ucb/text/kas087-006.pdf.
- ↑ Oldani, Michael J. (2004). "Thick Prescriptions: Toward an Interpretation of Pharmaceutical Sales Practices". Medical Anthropology Quarterly 18 (3): 325–356. doi:10.1525/maq.2004.18.3.325. ISSN 1548-1387. PMID 15484967.
- ↑ "Narrative review: the promotion of gabapentin: an analysis of internal industry documents". Annals of Internal Medicine 145 (4): 284–93. August 2006. doi:10.7326/0003-4819-145-4-200608150-00008. PMID 16908919.
- ↑ Henney JE (August 2006). "Safeguarding patient welfare: who's in charge?". Annals of Internal Medicine 145 (4): 305–7. doi:10.7326/0003-4819-145-4-200608150-00013. PMID 16908923.
- ↑ "Antiepileptics in migraine prophylaxis: An updated Cochrane review". Cephalalgia 35 (1): 51–62. August 2014. doi:10.1177/0333102414534325. PMID 25115844.
- ↑ "The 2012 AHS/AAN guidelines for prevention of episodic migraine: a summary and comparison with other recent clinical practice guidelines". Headache 52 (6): 930–45. June 2012. doi:10.1111/j.1526-4610.2012.02185.x. PMID 22671714.
- ↑ "Justice Department Announces Largest Health Care Fraud Settlement in Its History". United States Department of Justice. 2009-09-09. https://www.justice.gov/opa/pr/justice-department-announces-largest-health-care-fraud-settlement-its-history. "American pharmaceutical giant Pfizer Inc. and its subsidiary Pharmacia & Upjohn Company Inc. (hereinafter together "Pfizer") have agreed to pay $2.3 billion, the largest health care fraud settlement in the history of the Department of Justice, to resolve criminal and civil liability arising from the illegal promotion of certain pharmaceutical products, the Justice Department announced today."
- ↑ Harris, Gardiner (September 2, 2009). "Pfizer pays $2.3 billion to settle marketing case". The New York Times. https://www.nytimes.com/2009/09/03/business/03health.html.
- ↑ 192.0 192.1 Harris, Gardiner (September 3, 2009). "Pfizer Pays $2.3 billion to Settle Marketing Case". The New York Times. https://www.nytimes.com/2009/09/03/business/03health.html.
- ↑ 193.0 193.1 Johnson, Carrie (September 3, 2009). "In Settlement, A Warning To Drugmakers: Pfizer to Pay Record Penalty In Improper-Marketing Case". The Washington Post. https://www.washingtonpost.com/wp-dyn/content/article/2009/09/02/AR2009090201449_pf.html.
- ↑ 194.0 194.1 "Pfizer agrees record fraud fine". BBC News. September 2, 2009. http://news.bbc.co.uk/2/hi/business/8234533.stm.
- ↑ "Corporate Integrity Agreement between the Office of the Inspector General of the Department of Health and Human Services and Pfizer Inc.". August 31, 2009. https://oig.hhs.gov/fraud/cia/agreements/pfizer_inc.pdf.
- ↑ "ROST v. PFIZER, INC.". Casetext. https://casetext.com/case/rost-v-pfizer-2.
- ↑ Berenson, Alex (June 8, 2005). "At Pfizer, the Isolation Increases for a Whistle-Blower". The New York Times. https://www.nytimes.com/2005/06/08/business/at-pfizer-the-isolation-increases-for-a-whistleblower.html.
- ↑ Staton, Tracy (June 14, 2010). "Congress joins probe into Wyeth's Rapamune marketing". Fierce Pharma. https://www.fiercepharma.com/pharma/congress-joins-probe-into-wyeth-s-rapamune-marketing.
- ↑ Palmer, Eric (June 14, 2010). "Pfizer settles more off-label marketing cases tied to Rapamune". Fierce Pharma. https://www.fiercepharma.com/regulatory/pfizer-settles-more-off-label-marketing-cases-tied-to-rapamune.
- ↑ Edwards, Jim (June 10, 2010). "Blue Cross Names and Shames Pfizer Execs Linked to Massages-for-Prescriptions Push". CBS News. https://www.cbsnews.com/news/blue-cross-names-and-shames-pfizer-execs-linked-to-massages-for-prescriptions-push/.
- ↑ Bounds, Jeff (June 10, 2010). "Blue Cross Blue Shield of Texas sues Pfizer". American City Business Journals. https://www.bizjournals.com/austin/stories/2010/06/07/daily52.html.
- ↑ Staton, Tracy (June 11, 2010). "BCBS names Pfizer managers in kickback suit". Fierce Pharma. https://www.fiercepharma.com/pharma/bcbs-names-pfizer-managers-kickback-suit.
- ↑ "Blue Cross Blue Shield of Texas sues Pfizer over drug marketing". The Dallas Morning News. June 11, 2010. https://www.dallasnews.com/business/2010/06/11/blue-cross-blue-shield-of-texas-sues-pfizer-over-drug-marketing/.
- ↑ 204.0 204.1 Griffin, Drew; Segal, Andy (April 2, 2010). "Feds found Pfizer too big to nail". CNN. https://edition.cnn.com/2010/HEALTH/04/02/pfizer.bextra/index.html.
- ↑ Smythe, Christie (June 2, 2014). "Pfizer Agrees to $325 Million Neurontin Marketing Accord". Bloomberg News. https://www.bloomberg.com/news/2014-06-02/pfizer-agrees-to-325-million-settlment-over-neurontin.html.
- ↑ Kary, Tiffany (June 27, 2013). "Pfizer to pay $958M to end asbestos litigation". Bloomberg News. https://www.theday.com/article/20130627/BIZ03/306279581.
- ↑ Meier, Barry (July 2, 1994). "Pfizer Unit to Settle Charges Of Lying About Heart Valve". The New York Times. https://www.nytimes.com/1994/07/02/business/pfizer-unit-to-settle-charges-of-lying-about-heart-valve.html.
- ↑ "Ex-Pfizer Worker Cites Genetically Engineered Virus In Lawsuit Over Firing". Hartford Courant. March 14, 2010. https://www.courant.com/news/connecticut/hc-xpm-2010-03-14-hc-pfizer-virus-lawsuit-0314-artmar14-story.html.
- ↑ Pollack, Andrew; Wilson, Duff (April 2, 2010). "A Pfizer Whistle-Blower Is Awarded $1.4 Million". The New York Times. https://www.nytimes.com/2010/04/03/business/03pfizer.html.
- ↑ "Pfizer Settles B.Y.U. Lawsuit Over Development of Celebrex". The New York Times. Associated Press. May 1, 2012. https://www.nytimes.com/2012/05/02/health/pfizer-settles-byu-lawsuit-over-development-of-celebrex.html.
- ↑ Oldani, Michael (2016), "Trovafloxacin (Trovan) Controversy", The SAGE Encyclopedia of Pharmacology and Society, SAGE Publications, Inc., pp. 1444–1447, doi:10.4135/9781483349985.n409, ISBN 9781483350004, http://sk.sagepub.com/reference/the-sage-encyclopedia-of-pharmacology-and-society/i11727.xml, retrieved 2019-01-21
- ↑ Murray, Senan (June 20, 2007). "Anger at deadly Nigerian drug trials". BBC News. http://news.bbc.co.uk/2/hi/africa/6768799.stm.
- ↑ "Sequelae due to bacterial meningitis among African children: a systematic literature review". BMC Medicine 7: 47. 2009. doi:10.1186/1741-7015-7-47. PMID 19751516.
- ↑ "Nigerians sue Pfizer over test deaths". BBC News. August 30, 2001. http://news.bbc.co.uk/2/hi/business/1517171.stm.
- ↑ 215.0 215.1 "WikiLeaks Cables: Pfizer Targeted Nigerian Attorney General to Undermine Suit over Fatal Drug Tests". Democracy Now!. December 17, 2010. https://www.democracynow.org/2010/12/17/wikileaks_cables_pfizer_targeted_nigerian_attorney.
- ↑ "Panel Faults Pfizer in '96 Clinical Trial In Nigeria". The Washington Post. May 7, 2006. https://www.washingtonpost.com/wp-dyn/content/article/2006/05/06/AR2006050601338.html.
- ↑ Edwards, Jim (February 10, 2011). "Pfizer Bribed Nigerian Officials in Fatal Drug Trial, Ex-Employee Claims". CBS News. https://www.cbsnews.com/news/pfizer-bribed-nigerian-officials-in-fatal-drug-trial-ex-employee-claims/.
- ↑ "Trovan, Kano State Civil Case – Statement Of Defense". Pfizer. July 2007. https://cdn.pfizer.com/pfizercom/news/trovan_statement_defense_summary.pdf.
- ↑ "Pfizer-Nigeria appeal dismissed". BBC News. June 29, 2010. http://news.bbc.co.uk/2/hi/world/us_and_canada/10454982.stm.
- ↑ Boseley, Sarah (December 9, 2010). "WikiLeaks cables: Pfizer 'used dirty tricks to avoid clinical trial payout'". The Guardian (London). https://www.theguardian.com/business/2010/dec/09/wikileaks-cables-pfizer-nigeria.
- ↑ "Pfizer Statement Regarding Article In The Guardian" (PDF) (Press release). Pfizer. December 9, 2010.
- ↑ Edwards, Jim (January 4, 2011). "In Defense of Blackmail: Why Shouldn't Pfizer Dig Dirt on Crooked Pols?". CBS News. https://www.cbsnews.com/news/in-defense-of-blackmail-why-shouldnt-pfizer-dig-dirt-on-crooked-pols/.
- ↑ "Michael Aondoakaa "Unfit" To Remain SAN, Says CDHR In High-Powered Petition". http://saharareporters.com/2010/07/19/michael-aondoakaa-%E2%80%9Cunfit%E2%80%9D-remain-san-says-cdhr-high-powered-petition.
- ↑ "Wikileaks on Nigeria's Corrupt Oil Sales at NNPC, Shell, US Ambassador". December 12, 2010. http://usafricaonline.com/2010/12/12/wikileaks-on-nigerias-oil-corruption-nnpc-shell-us-ambassador-attorney-general-aondoakaa/.
- ↑ Lenzer, J. (16 August 2011). "Pfizer settles with victims of Nigerian drug trial". BMJ 343 (aug16 3): d5268. doi:10.1136/bmj.d5268. PMID 21846712.
- ↑ Browning, Jonny (21 July 2022). "Pfizer, Flynn Fined £70 Million for Epilepsy Drug Prices". Bloomberg. https://www.bloomberg.com/news/articles/2022-07-21/pfizer-flynn-fined-70-million-for-unfair-epilepsy-drug-prices#xj4y7vzkg.
- ↑ Reed, Jim (2022-08-26). "Moderna suing Pfizer over Covid vaccine technology". BBC News. https://www.bbc.com/news/health-62691102.
- ↑ "Pfizer Implemented More than 4,000 Greenhouse Gas Reduction Projects Since 2000". United States Chamber of Commerce. November 15, 2019. https://www.uschamber.com/article/pfizer-implemented-more-4000-greenhouse-gas-reduction-projects-2000.
- ↑ "Pfizer Recognized by Carbon Disclosure Project for Carbon Performance" (Press release). Pfizer. September 13, 2012 – via Business Wire.
- ↑ "American Cyanamid Superfund Site Fact Sheet". December 2011. https://www.nj.gov/dep/srp/community/sites/pi/american_cyanamid_fs.pdf.
- ↑ Paik, Eugene (March 9, 2012). "Activists say EPA $204M fix for polluted American Cyanamid property will not permanently resolve problem". NJ.com. https://www.nj.com/news/2012/03/activisits_say_epa_fix_for_pol.html.
- ↑ "The tempest". The Washington Post. May 28, 2006. https://www.washingtonpost.com/wp-dyn/content/article/2006/05/23/AR2006052301305_pf.html.
- ↑ Simonyi, Charles; Dijkgraaf, Robbert (2018). "Report for the Academic Year 2017-2018". https://www.ias.edu/sites/default/files/documents/publications/Annual%20Report%2017%E2%80%9318%20web.pdf.
- ↑ Simonyi, Charles; Dijkgraaf, Robbert (2014). "Report for the Academic Year 2013-2014". https://www.ias.edu/sites/default/files/documents/publications/annualreport1314_0.pdf.
- ↑ "Donor Listing" (in en). https://boundless.utoronto.ca/our-supporters/donor-listing/.
- ↑ "Presidents' Circle Member Listing | Ways to Give". 2022-05-01. https://engage.utoronto.ca/site/SPageServer?pagename=presidents_circle_member_listing.
- ↑ "Honor Roll of Donors". October 2020. https://uw-s3-cdn.s3.us-west-2.amazonaws.com/wp-content/uploads/sites/83/2020/10/29094302/RTC_HonorRoll_2020.pdf.
- ↑ Hewitt, Bradford L.; Reckford, Jonathan T.M. (2021-11-15). "Annual Report FY2021". https://www.habitat.org/media/7101/download.
- ↑ "Corporate Partners". https://www.hrc.org/about/corporate-partners.
- ↑ "Annual Report FY 2015-2016". 2016. https://nwlc.org/wp-content/uploads/2021/11/final_FY2015-2016_NWLC_AnnualReport.pdf.
- ↑ Shore, Billy; Nelson, Tom (2017). "No Kid Hungry 2017". https://www.shareourstrength.org/wp-content/uploads/2018/05/AnnualReport_2017_mid.pdf.
- ↑ "Investments in Health - Pfizer 2010 Annual Review". 2011. https://www.pfizer.com/sites/default/files/investors/financial_reports/annual_reports/2010/access-investments.html.
- ↑ "Sponsors - Past Sponsors" (in en-US). https://www.womeninmedicinesummit.org/sponsors-past-sponsors.
- ↑ "Sponsors" (in en-US). https://neuroscienceinnovationforum.org/sponsors/.
- ↑ Snyder Bulik, Beth (2021-02-24) (in en). 'Mission Possible': Pfizer and BioNTech star in their own vaccine discovery movie. https://www.fiercepharma.com/marketing/mission-possible-pfizer-and-biontech-star-vaccine-discovery-movie. Retrieved 2022-04-08.
- ↑ "The Power of Philanthropy". 2016. https://media.nationalgeographic.org/assets/file/2016_Annual_Report_DONOR_LIST_FINAL.pdf.
- ↑ Adams, Ben (2022-03-28). "Pfizer and BioNTech go to Hollywood with splashy Oscars sponsorship" (in en). Fierce Pharma. https://www.fiercepharma.com/marketing/pfizer-and-biontech-take-hollywood-they-sponsor-oscars. Retrieved 2022-05-23.
- ↑ "National Press Foundation". 2013. http://nationalpress.org/about/our-funders/.
- ↑ "Our Funders". 2010. http://nationalpress.org/about/our-funders/.
- ↑ "Cancer Issues 2010". 2010. http://nationalpress.org/programs-and-resources/program/cancer-issues-2010/.
- ↑ Raeburn, Paul (2010-10-13). "Cover this great cancer conference! (Yes, there's a catch...)" (in en-US). https://ksj.mit.edu/tracker-archive/cover-great-cancer-conference-yes-theres/.
- ↑ "Who We Are". 2021-06-03. https://www.19tozero.ca/who-we-are.
- ↑ "Corporate Support" (in en). https://www.hematology.org/about/corporate-support.
- ↑ "Partners". https://arthritis.ca/about-us/partners.
- ↑ "Our Corporate Partners". https://cancer.ca/en/get-involved/partnerships/our-corporate-partners.
- ↑ 256.0 256.1 Public Health Agency of Canada (2022-02-25). "National Advisory Committee on Immunization (NACI): Membership and representation". https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/naci-membership-representation.html.
- ↑ "Sponsors" (in en-US). https://csim.ca/conferences/virtual-annual-meeting-2021/sponsors/.
- ↑ "Corporate Liaison Board" (in en). https://www.endocrine.org/membership/corporate-liaison-board.
- ↑ "Why sponsor the EURObservational Registry Programme". https://www.escardio.org/Research/Registries-%26-surveys/Sponsorship%2C%20https%3A//www.escardio.org/Research/Registries-%26-surveys/Sponsorship.
- ↑ "Sociedad Española de Cardiología: profesionales sanitarios y cardiólogos". https://secardiologia.es/.
- ↑ "AVAC Member Organizations" (in en-US). 2021. https://adultvaccinesnow.org/our-membership/avac-member-organizations/.
- ↑ "List of Members" (in en-US). 2022-03-04. https://www.strengthenfda.org/members.
- ↑ "Our Members" (in en-US). 2017. https://www.amrindustryalliance.org/our-members/.
- ↑ "Member Listings" (in en-US). 2021-12-23. https://www.biotech.ca/about/member-listings/.
- ↑ Wilson, Barry (2000-10-05). "Biotech lobby group at heart of ethics complaint" (in en). https://www.producer.com/news/biotech-lobby-group-at-heart-of-ethics-complaint/.
- ↑ "2020-2021 Annual Report". 2021. https://bipartisanpolicy.org/download/?file=/wp-content/uploads/2021/06/BPC-Annual-Report_6.8.pdf.
- ↑ "Active Members". 2022. https://businesscouncil.com/active-members/.
- ↑ "Members" (in en-US). https://www.businesscouncilfortheun.org/bcun-members.
- ↑ "2013 Foundation and Institutional Support". 2013. https://www.cbpp.org/sites/default/files/atoms/files/foundationlist2013final.pdf.
- ↑ Dean, Arthur T. (2015). "2015 Annual Report". http://2015.cadcaannualreport.org/CADCA-2015-AnnualReport.pdf.
- ↑ "About The Project" (in en-US). 2020. https://covidvaccineproject.org/about-the-project/.
- ↑ "Membership". 2022. https://www.efpia.eu/about-us/membership/.
- ↑ "2020 Donors" (in en). https://2020-annual-report.fnih.org/donors/.
- ↑ "Members" (in en-US). https://globalhealth.org/about-us/members/.
- ↑ "About Us" (in en-AU). https://www.immunisationcoalition.org.au/about-us/.
- ↑ "Member Companies" (in en). http://innovativemedicines.ca/about/member-companies/.
- ↑ Grant, Kelly (2019-12-11). "Innovative Medicines Canada bans members from paying doctors fees for IV infusions" (in en-CA). The Globe and Mail. https://www.theglobeandmail.com/canada/article-innovative-medicines-canada-bans-members-from-paying-doctors-fees-for/.
- ↑ "Consultant Lobbyist Registration No. CL4899-20200917025456". 2021-09-22. https://lobbyist.oico.on.ca/Pages/Public/PublicSearch/.
- ↑ Maher, Stephen (2019-01-29). "For access to the Ford government, two names matter most". https://www.macleans.ca/politics/for-access-to-the-ford-government-two-names-matter-most/.
- ↑ "Innovative Medicines Canada/Médicaments Novateurs Canada / Andrew Balfour, Consultant". 2022-03-28. https://lobbycanada.gc.ca/app/secure/ocl/lrs/do/clntSmmry?clientOrgCorpNumber=367878&sMdKy=1621857998861.
- ↑ "Companies" (in en-GB). https://www.ifpma.org/who-we-are/our-membership/full-members/companies/.
- ↑ "LifeSciences British Columbia Pfizer Announcement". 2007-10-18. https://vitp.ca/post417/.
- ↑ "Our Sponsors" (in en-US). January 6, 2021. https://lifesciencesbc.ca/membership/our-sponsors/.
- ↑ "Our Members" (in en-US). https://nationalhealthcouncil.org/members/.
- ↑ "Members" (in en). https://www.npcnow.org/about/members.
- ↑ "Current Members" (in en). https://www.personalizedmedicinecoalition.org/Members/Current_Members.
- ↑ "SHOWCASE: Precision Medicine: Expanding the Frontiers of Precision Medicine" (in en-US). 2021-04-01. https://www.pharmavoice.com/news/2021-04-precision-medicine/612084/.
- ↑ "Our Organization" (in en). https://www.paab.ca/about.htm.
- ↑ "About". https://www.phrma.org/about.
- ↑ Schwartz, Brian (2021-06-01). "Big Pharma lobbyists launch campaign against Biden over Covid vaccine patent waiver" (in en). https://www.cnbc.com/2021/06/01/big-pharma-launches-campaign-against-biden-over-covid-vaccine-patent-waiver.html.
- ↑ "2021 Annual Report". 2021. https://reaganudall.org/about-us/annual-reports/2021-annual-report.
- ↑ "Member Organizations" (in en). 2007-09-28. https://www.researchamerica.org/about-us/member-organizations.
- ↑ "U.S. Global Leadership Coalition, Global Trust members". U.S. Global Leadership Coalition. https://www.usglc.org/about-us/coalition-members/.
- ↑ Ivan, Wecke (2021-08-16). "Conspiracy theories aside, there is something fishy about the Great Reset" (in en). https://www.opendemocracy.net/en/oureconomy/conspiracy-theories-aside-there-something-fishy-about-great-reset/.
- ↑ "Pfizer" (in en). https://www.weforum.org/organizations/pfizer/.
- ↑ Mishra, Manas (July 2, 2019). "Senator Warren asks former FDA chief Gottlieb to resign from Pfizer board". Reuters. https://www.reuters.com/article/us-pfizer-board-scott-gottlieb/senator-warren-asks-former-fda-chief-gottlieb-to-resign-from-pfizer-board-idUSKCN1TX2IX.
- ↑ "Registration ID: 200777" (in en). 2012-04-10. https://www.lobbyistsregistrar.bc.ca/app/secure/orl/lrs/do/vwRg?cno=2406®Id=200777.
- ↑ "US Medical, Scientific, Patient and Civic Organization Funding Report: Q1-Q2 2021". Pfizer. 2021-09-27. https://s3.documentcloud.org/documents/23787007/pfizer-2021-report.pdf.
- ↑ "ASA Organizational Members". https://ww2.amstat.org/membership/corporatesupporters.cfm.
- ↑ "Sponsors" (in en-ca). 2021. https://www.biomb.ca/members/sponsors.
- ↑ "Our Sponsors and Prizes". https://www.bcpharmacy.ca/conference/prizes/2022.
- ↑ "Patrons". 2010-07-14. https://www.cacmid.ca/.
- ↑ "Annual Report 2018-2019". 2019. https://caep.ca/wp-content/uploads/2019/06/Annual-Report2019-V2.pdf.
- ↑ "Pfizer Sponsor Showcase". https://camo-acom.ca/Pfizer-Sponsor-Showcase.
- ↑ Weeks, Carly (2009-12-02). "Medical association takes heat for Pfizer funding" (in en-CA). The Globe and Mail. https://www.theglobeandmail.com/life/health-and-fitness/medical-association-takes-heat-for-pfizer-funding/article568583/.
- ↑ "Meet Our Sponsors" (in en). Canadian Pharmacists Journal / Revue des Pharmaciens du Canada 147 (3): 191–192. May 2014. doi:10.1177/1715163514530860. ISSN 1715-1635. PMID 24847373.
- ↑ "2018 Annual Report". https://www.cpha.ca/sites/default/files/uploads/about/reports/2018-annual-report-web-e.pdf.
- ↑ "2020-2021 Annual Report: Collaboration, Resilience and Advancement". January 2022. https://rheum.ca/wp-content/uploads/2022/01/CRA_20-21_AnnualReport_EN.pdf.
- ↑ "Home". 2021. https://cuameeting.org/en/.
- ↑ McLorie, Gordon (2000). "Fall 2000 Newsletter". http://history.cua.org/sites/default/files/2018-07/2000-02_en.pdf.
- ↑ "Your impact". 2022. https://www.oma.org/what-we-do/ontario-medical-foundation/your-impact/.
- ↑ "Pfizer Canada - Platinum Sponsor". https://pans.ns.ca/pfizer-canada-platinum-sponsor.
- ↑ "Thank you to our donors". 2021. https://www.camh.ca/-/media/files/camhf-2020-21-annual-report-donor-listing.pdf.
- ↑ "2012 Honor Roll of Donors". https://www.pageturnpro.com/Dana-Farber-Cancer-Institute-and-The-Jimmy-Fund/49214-2012-Honor-Roll-of-Donors/sdefault.html#page/8.
- ↑ "AGM, Leadership & Advocacy Workshops 09". 2009. https://fmwc.ca/wp-content/uploads/2013/12/FMWC_Fall_2009_issue.pdf.
- ↑ "Sponsors & Partners". https://fmwc.ca/about-us/sponsors-partners/.
- ↑ "Impact report 2020: Pivoting with the pandemic". 2021. https://foodallergycanada.ca/wp-content/uploads/FAC-ImpactReport-2020.pdf.
- ↑ "Who we work with" (in en-US). https://foodallergycanada.ca/our-impact-advocacy-and-services/about-us/who-we-work-with/.
- ↑ "2020-21 SickKids Annual Report". 2021. https://web.sickkidsfoundation.com/annual-report-2020/.
- ↑ "Partners". 2016. http://www.medicalteams.org/take-action/corporate-partnerships/partners/humanitarian-aid-donors.
- ↑ "Donor List". 2019. https://nbrhc.on.ca/wp-content/uploads/2019/12/2019-Donor-Wall-List.doc.
- ↑ "The 8th Princess Margaret Hospital Conference: Developments in Cancer Management" (in en). https://fdocuments.in/document/years-of-success-anna-greenberg-richard-hill-michael-jewett-david-mccready-hans.html.
- ↑ "2021 Annual Report". 2021. https://thepmcf.ca/getmedia/9b96649e-83ba-490a-bacc-f961904b94c8/Annual-Report-2021_Digital.pdf.
- ↑ "Our Donors" (in en-CA). 2021. https://www.shnfoundation.ca/our-donors/.
- ↑ "Donor List - 2020-2021". https://www.sinaihealthannualreport.ca/donor-list-2020-2021.
- ↑ "Our donors - Your Impact" (in en). 2022. https://sunnybrook.ca/foundation/your-impact/our-donors-2021.html.
- ↑ "Recognizing Your Contribution" (in en-CA). 2022. https://www.uhkf.ca/Your-Impact/Recognizing-Your-Contribution.
- ↑ "Holi Gala Festival of Colours 2022". https://support.oslerfoundation.org/site/SPageServer?pagename=holigala22_our_sponsors.
- ↑ Bettinger, Julie (2020-01-24). "Vaccine Hesitancy: It doesn't matter if the vaccine works if nobody gets it". https://mediasite.phsa.ca/Mediasite/Catalog/Mobile/FolderPresentation/22912938f963448cab10cf3719de3d5e21/22912938f963448cab10cf3719de3d5e21/b27eb1456cb14e06af5370550b1c5e431d/.
- ↑ "Corporate Sponsors of RMHC" (in en). https://rmhc.org/about-us/our-partners.
- ↑ "Our Sponsors" (in en-US). 2022. https://www.abcglobalalliance.org/who-we-are/our-supporters/.
- ↑ "Members". 2014-07-13. http://advamed.org/page/33/members.
- ↑ "Advancing Gene, Cell, & Tissue-Based Therapies" (in en). 2019. https://alliancerm.org/sector-report/2019-annual-report/.
- ↑ "Our Supporters" (in en-AU). https://arthritisaustralia.com.au/about-us/our-supporters/.
- ↑ "We are very honoured to count Pfizer among the Sponsors of BioFIT 2021" (in en-GB). 2021-11-29. https://www.biofit-event.com/we-are-very-honoured-to-count-pfizer-among-the-sponsors-of-biofit-2021/.
- ↑ "Industry and Association Partners" (in en-US). https://www.cfn-nce.ca/get-involved/our-partners/industry-and-association-partners/.
- ↑ Kim, Perry (2020-04-07). "Request for Proposals (RFP) Frailty and COVID-19". https://www.cfn-nce.ca/wp-content/uploads/2020/04/RFP-Frailty-and-COVID-April-7.pdf.
- ↑ "Our Partnerships" (in en). 2021. https://www.colorectalcancercanada.com/what-we-do/our-partnerships/.
- ↑ "In Conversation with Barry Stein" (in en). 2022-02-28. https://www.pfizer.ca/conversation-barry-stein.
- ↑ "Our partners" (in en-GB). 2020-04-24. https://dndi.org/partnerships/industry-partners/.
- ↑ "Grants and Donations". 2022. https://www.gisaid.org/about-us/grants-and-donations/.
- ↑ "Our partners" (in en). https://www.heartandstroke.ca/en/what-we-do/partners/.
- ↑ "Pfizer Canada announces support to Heart & Stroke's #TimeToSeeRed campaign" (in en). 2018-04-27. https://www.pfizer.ca/pfizer-canada-announces-support-heart-stroke%E2%80%99s-timetoseered-campaign.
- ↑ "Our Partners" (in en-US). https://lunghealth.ca/our-partners/.
- ↑ "Sponsors". https://www.mentoringinibd.com/about-us/sponsors/.
- ↑ "COVID-19 Cohort Study (CCS): Study of the epidemiology of COVID-19 in healthcare workers and their households" (in en). https://www.tibdn.ca/covid-19/sinai.
- ↑ "Response Package HTH-2015-51828". 2016-02-12. http://docs.openinfo.gov.bc.ca/Response_Package_HTH-2015-51828.pdf.
- ↑ Ganton, Jennifer (2015-10-22). "Drugs commonly used in kidney transplant patients not as effective as previously thought". https://www.ohri.ca//newsroom/story/view/622?l=en.
- ↑ "Sponsors and CRO's" (in en-US). http://www.pinnacletrials.com/sponsors/.
- ↑ "A-Z". 2022. https://www.radcliffecardiology.com/products-services/a-z.
- ↑ "Featured partnerships" (in en). 2022. https://truthinitiative.org/what-we-do/partnerships/featured-partnerships.
External links
- Business data for Pfizer Inc.:
- Pfizer Inc. recipient profile on USAspending.gov
 |
 KSF
KSF