Ice III
Topic: Earth
 From HandWiki - Reading time: 3 min
From HandWiki - Reading time: 3 min
Short description: Alternative state of water ice
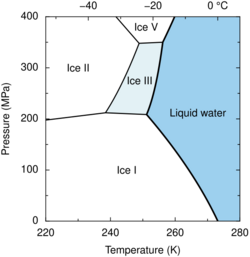
Ice III is a form of solid matter which consists of tetragonal crystalline ice, formed by cooling water down to 250 K at 300 MPa. It is the least dense of the high-pressure water phases, with a density of 1160 kg/m3 (at 350 MPa).[1] It has a very high relative permittivity at 117 and has a density of 1.16 g/cm3 (making it more dense than water). The proton-ordered form of ice III is ice IX.[2]
Ordinary water ice is known as ice Ih, (in the Bridgman nomenclature). Different types of ice, from Ice II to Ice XIX, have been created in the laboratory at different temperatures and pressures.
See also
- Ice, for other crystalline forms of ice
References
- ↑ "Ice III (ice-three) structure". 2012-02-04. http://www.lsbu.ac.uk/water/ice_iii.html.
- ↑ doktorholz (2018-12-17). "Ice III and IX" (in en). https://crystalsymmetry.wordpress.com/2018/12/17/ice-iii-and-ix/.
Licensed under CC BY-SA 3.0 | Source: https://handwiki.org/wiki/Earth:Ice_III15 views | ↧ Download this article as ZWI file
 KSF
KSF