Inhaler
Topic: Engineering
 From HandWiki - Reading time: 13 min
From HandWiki - Reading time: 13 min
| Inhaler | |
|---|---|
 Metered-dose inhaler (MDI) | |
| Specialty | pulmonology |
An inhaler (puffer, asthma pump or allergy spray) is a medical device used for delivering medicines into the lungs through the work of a person's breathing. This allows medicines to be delivered to and absorbed in the lungs, which provides the ability for targeted medical treatment to this specific region of the body, as well as a reduction in the side effects of oral medications. There are a wide variety of inhalers, and they are commonly used to treat numerous medical conditions with asthma and chronic obstructive pulmonary disease (COPD) being among the most notable.[1]
Some of the common types of inhalers include metered-dose inhalers, dry powder inhalers, soft mist inhalers, and nebulizers. Each device has advantages and disadvantages and can be selected based on individually specific patient needs, as well as age, pathological conditions, coordination, and lung function.[2] Proper education on inhaler use is important to ensure that inhaled medication creates its proper effects in the lungs.[3] Using a spacer can ensure that more medicine reaches the lungs, thus providing the most optimal treatment.
Medical uses
Inhalers are designed to deliver medication directly to the lungs through a person's own breathing. This may benefit a patient by providing medicines directly to areas of disease, allowing medication to take a greater effect on its intended target, and limit side effects of medications when administered locally.[1] Inhalers are used in a variety of different medical conditions with diseases of the lungs and respiratory system being among the most common. Individuals with these diseases/conditions need medications designed to decrease airway inflammation and obstruction to allow for easier and comfortable breathing.[4] Antibiotic medications have even been developed for inhalers to allow for direct delivery to areas of infection within the lungs.[5] Two of the most common conditions that warrant inhaler therapy are asthma and chronic obstructive pulmonary disease.[4][6]
Asthma
Asthma is a condition of intermittent airway obstruction due to inflammatory processes in the lungs. Inhaled medications are used to calm down the inflammation present in the lungs and allow for relief of the airway obstruction. Common inhaled medications used for treatment of asthma include long term inhalational steroidal anti-inflammatory drugs (most commonly inhaled corticosteroids, also called ICS) and fast-relieving bronchodilators such as salbutamol (known commonly as "Ventolin") and salmeterol. These medications allow for patients to have relief of airway obstruction symptoms and reduced inflammation.[4] If some people are unable to use inhalers, non-steroidal anti-inflammatory drugs (NSAIDs) may be used, but with caution since they may cause immunological hypersensitivity to NSAIDs, resulting in respiratory-related symptoms such as bronchospasms, acute asthma exacerbation, and severe asthma morbidity.[7][8]
Chronic obstructive pulmonary disease (COPD)
COPD is an obstructive lung disease due to long-term damage to the airways of the lungs. The long-term damage leads to the inability of the airways to open properly, causing airway obstruction. Inhaled medications allow patients to see improvement in symptoms and better function of daily living. Some commonly used inhaled medications in patient's with COPD are ipratroprium, salmeterol, and corticosteroids.[6] Inhalers that combine two or three different medications including inhaled corticosteroids, long-active muscarinic medications (LAMA) and long acting beta2 agonists (LABA) for treating COPD may be associated with improvements in some quality of life variables and small improvements in lung function and respiratory symptoms, however, may also be associated with an increase in the risk of pneumonia.[9]
Types of Inhalers
Meter-dosed inhaler (MDI)
The most common type of inhaler is the pressurifzed metered-dose inhaler (MDI) which is made up of 3 standard components- a metal canister, plastic actuator, and a metering valve. The medication is typically stored in solution in a pressurized canister that contains a propellant or suspension. The MDI canister is attached to a plastic, hand-operated actuator. On activation, the metered-dose inhaler releases a fixed dose of medication in aerosol form through the actuator and into a patient's lungs.[10] These devices require significant coordination as a person must discharge the medication at or near the same time that they inhale in order for the medication to be effective.[11]

Dry powder inhaler (DPI)
Dry powder inhalers release a metered or device-measured dose of powdered medication that is inhaled through a DPI device. This device usually contains a chamber in which the powdered medication is deposited prior to each dosage.[3] The powder can then be inhaled with a quick breath.[1] This allows for medication to be delivered to the lungs without the need for use of propellant/suspension.[11]
Soft mist inhaler (SMI)
Soft mist inhalers release a light mist containing medication without the need for a propellant/suspension. Upon pressing a button, the inhaler creates a mist of medication, allowing for inhalation into the lungs. SMIs suspend inhaled medications for roughly 1.2 seconds, which is longer than the average MDI inhaler suspension time period. This requires less coordination when using and may be helpful for young patients or patients that find the MDI inhalers difficult to use.[11]
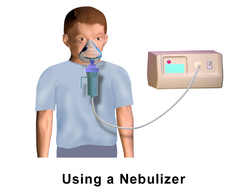
Nebulizer
Nebulizers are designed to deliver medications over an extended period of time over multiple breaths through a mouthpiece or face mask. They generate a continuous mist with aerosolized medication, allowing a patient to breathe normally and receive medications.[11] They are commonly used in infants and toddlers requiring inhaled medications or in patients in the hospital who require inhaled medications.[2]
Smart inhaler
The smart-inhaler is an inhaler that will automatically update an app with information that includes the time of day, air quality, and how many times it has been used through sensor technology on the device.[12] The first smart-inhaler was approved in 2019 by the FDA, its purpose is to track patient use of the device and some other circumstantial factors that could affect the effectiveness of the dosage.[12] This information is sent via Bluetooth to a mobile device app, and is later shared with their physician to determine what kind of things can trigger issues with asthma and other problems.[12] This technology presents a great way to cut down on medical costs associated with asthma and also help patients better manage their condition with fewer emergencies.
The Teva ProAir Digihaler was the first FDA approved smart inhaler.[13] It shows how effective the device is at aiding patients in using the proper dose amount for their asthma. In a study published by the European Respiratory Journal, the ProAir Digihaler accurately identified when patients were using their inhalers and whether they were effectively administering the dose in a 370 patient trial with the device.[13] This study further gives an overview on the technology regarding applications and devices that help aid in the tracking and medication management for asthma and other lung conditions. Another study showed that smart inhalers accurately recorded all doses administered by patients with their technology, which signifies their importance in providing accurate dosage information to patients and their physicians.[14]
Propellants
In 2009, the FDA banned the use of inhalers that use chlorofluorocarbons (CFC) as propellants. In their place, inhalers now use hydrofluoroalkane (HFA). HFA is not environmentally inert as it is a greenhouse gas but it does not affect the ozone layer.[15] While some people with asthma and advocacy groups contend that HFA inhalers are not as effective,[16] published clinical studies indicate CFC and HFA inhalers are equally effective in controlling asthma.[17]
While the impact of CFCs from inhalers on the ozone layer had been minuscule (dwarfed by industrial processes using CFCs), the FDA in its interpretation of the Montreal Protocol mandated the switch in propellants.[15] Patients expressed concern about the high price of the HFA inhalers as there were initially no generic versions, whereas generic CFC inhalers had been available.[16]
Proper use
It is important to use proper techniques when administering medications through inhalers.
Proper use of inhalers often involves initial deep breathing (which involves mostly the diaphragm's movements), and then rapid breathing[18] (which involves most of the muscles of respiration, such as external and internal intercostal muscles[19]) during intake of one or more puffs from the inhalers.
Improper use of inhalers is very common, can lead to distribution of the medicine into the mouth or throat where it cannot create its desired effect and may cause harm.[1][20][21] Education on the correct use of inhalers for delivery of medications is a commonly cited topic in medical studies and a great deal of thought has been put into how best to help people learn to use their inhalers effectively.[22][3] Below is a description of proper inhaler technique for each different type of inhaler as well as a helpful video explaining what the text states.
Meter-dosed inhalers
1. The mouthpiece is removed and the inhaler is shaken for 5–10 seconds
2. The inhaler is gripped with mouthpiece on the bottom and canister on top. A finger is placed on the canister to allow for delivery of medicine.
3. Deep inhalation is done until no more air can be taken into the lungs.
4. Deep exhalation is done until most of the air is out of the lungs.
5. Once deep exhalation is done, mouth is placed over mouthpiece.
6. As the next deep inhalation begins, the canister is pressed down to release the medicine into the lungs.
5. Slow deep breathing is continued and breath is held for 5–10 seconds, keeping the medicine in the lungs for a longer time period and preventing escape of aerosolized form of the medicine.
6. Complete exhalation is done again. If multiple puffs of the medicine have to be taken, steps 1–5 are repeated after waiting for 15–30 seconds.
7. Mouthpiece is replaced.[1]
With spacer
Spacer is placed at the mouthpiece of a meter-dosed inhaler while keeping mouth at the end of the spacer. Deep breathing is done to be ready for the delivery of the medicine to the lungs, and then the canister is pressed down. This minimizes need for coordination of breathing with inhaler activation.[1]
Dry powder inhalers
1. Inhaler medication chamber is prepared (this will be different based on the type of inhaler but will involve preparing and opening the chamber with the medication)
2. The inhaler is held with the chamber pointing towards the patient and complete exhalation is done with their head turned away from the inhaler.
3. Mouth is placed over the chamber and a quick, deep breath is taken allowing medication to dispense in the lungs.
4. Breath is held for 5–10 seconds and then slow exhalation is done.
5. After waiting for a few minutes, steps 1-4 are repeated if another dose is needed.[1]
Soft mist inhalers
1. The inhaler is primed by loading the cartridge and discharging the inhaler until a fine mist is visible (more explanation in the video).
2. Once complete exhalation is done, mouth is placed around the mouthpiece while leaving space for the small holes on the side of the mouthpiece.
3. Slow inhalation is done while simultaneously pressing the button to release the medication.
4. Breath is held for 5–10 seconds.
5. Slow exhalation is done and steps 1-4 are repeated if another dose of medication is required after waiting for a few minutes.
If inhaler is used everyday, the inhaler usually has to be primed the first time using a new cartridge, but it may need to be primed again if it has not been used in multiple days.[1]
After use
If using inhaled corticosteroids, rinse mouth out directly after use of inhaler. This helps to prevent infection.[1]
Nebulizer
1. Mouth is placed over mouthpiece or face mask is placed over nose and mouth
2. The nebulizer machine is turned on.
3. Normal breathing is done for 10-20 min (or time allotted for treatment).
4. Machine is turned off and face mask/mouthpiece remove is removed.[1]
Price and availability
In the United States, pharmaceutical manufacturers use legal and regulatory strategies to keep inhaler prices artificially high. There has been little innovation in inhaler technology for decades — the most recent drug to be approved by the FDA for treating asthma or COPD via a novel target of action was Ipratropium bromide in 1986. Since then, manufacturers have used small changes to drug delivery mechanisms, or have switched active ingredients from one inhaler device to another (a strategy known as a "device hop") to keep patents active. This has the effect of limiting competition, keeping inhalers expensive.[23] Because of high prices, patients sometimes skip doses or give up using their inhalers.
History
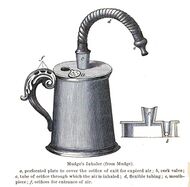
The idea of directly delivering medication into the lungs was based on ancient traditional cures that involved the use of aromatic and medicinal vapors. These did not involve any special devices beyond the apparatus used for burning or heating to produce fumes. Early inhalation devices included one devised by John Mudge in 1778. It had a pewter mug with a hole allowing attachment of a flexible tube. Mudge used it for the treatment of coughs using opium. These devices evolved with modifications by Wolfe, Mackenzie (1872) and better mouth attachments such as by Beigel in 1866. Many of these early inhalers needed heat to vaporize the active chemical ingredient. The benefits of forced expiration and inspiration to treat asthma were noted by J. S. Monell in 1865. Chemicals used in inhalers included ammonia, chlorine, iodine, tar, balsams, turpentine camphor and numerous others in combinations.[24] Julius Mount Bleyer used a variation in 1890 in New York.[25]
In 1968, Robert Wexler of Abbott Laboratories developed the Analgizer, a disposable inhaler that allowed the self-administration of methoxyflurane vapor in air for analgesia.[26] The Analgizer consisted of a polyethylene cylinder 5 inches long and 1 inch in diameter with a 1 inch long mouthpiece. The device contained a rolled wick of polypropylene felt which held 15 milliliters of methoxyflurane.
Because of the simplicity of the Analgizer and the pharmacological characteristics of methoxyflurane, it was easy for patients to self-administer the drug and rapidly achieve a level of conscious analgesia which could be maintained and adjusted as necessary over a period of time lasting from a few minutes to several hours. The 15 milliliter supply of methoxyflurane would typically last for two to three hours, during which time the user would often be partly amnesic to the sense of pain; the device could be refilled if necessary.[27]
The Analgizer was found to be safe, effective, and simple to administer in obstetric patients during childbirth, as well as for patients with bone fractures and joint dislocations,[27] and for dressing changes on burn patients.[28] When used for labor analgesia, the Analgizer allows labor to progress normally and with no apparent adverse effect on Apgar scores.[27] All vital signs remain normal in obstetric patients, newborns, and injured patients.[27] The Analgizer was widely utilized for analgesia and sedation until the early 1970s, in a manner that foreshadowed the patient-controlled analgesia infusion pumps of today.[29][30][31][32] The Analgizer inhaler was withdrawn in 1974, but use of methoxyflurane as a sedative and analgesic continues in Australia and New Zealand in the form of the Penthrox inhaler.[33][34][35][36][37][38]
See also
- List of medical inhalants
- Decongestant
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 National Asthma Education Prevention Program (November 2007). "Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007". The Journal of Allergy and Clinical Immunology 120 (5 Suppl): S94-138. doi:10.1016/j.jaci.2007.09.043. PMID 17983880.
- ↑ 2.0 2.1 "Inhalation device options for the management of chronic obstructive pulmonary disease". Postgraduate Medicine 130 (1): 83–97. January 2018. doi:10.1080/00325481.2018.1399042. PMID 29210318.
- ↑ 3.0 3.1 3.2 "Inhaler Technique Education and Exacerbation Risk in Older Adults with Asthma or Chronic Obstructive Pulmonary Disease: A Meta-Analysis". Journal of the American Geriatrics Society 67 (1): 57–66. January 2019. doi:10.1111/jgs.15602. PMID 30291745.
- ↑ 4.0 4.1 4.2 "Diagnosis and Management of Asthma - The Swiss Guidelines". Respiration; International Review of Thoracic Diseases 95 (5): 364–380. 2018. doi:10.1159/000486797. PMID 29614508.
- ↑ "Inhaled colistin monotherapy for respiratory tract infections in adults without cystic fibrosis: a systematic review and meta-analysis". International Journal of Antimicrobial Agents 51 (1): 1–9. January 2018. doi:10.1016/j.ijantimicag.2017.05.016. PMID 28669836.
- ↑ 6.0 6.1 "Diagnosis, Prevention and Treatment of Stable COPD and Acute Exacerbations of COPD: The Swiss Recommendations 2018". Respiration; International Review of Thoracic Diseases 96 (4): 382–398. 2018. doi:10.1159/000490551. PMID 30138943.
- ↑ Lo, Pei-Chia; Tsai, Yueh-Ting; Lin, Shun-Ku; Lai, Jung-Nien (14 October 2016). "Risk of asthma exacerbation associated with nonsteroidal anti-inflammatory drugs in childhood asthma". Medicine 95 (41): e5109. doi:10.1097/MD.0000000000005109. ISSN 0025-7974. PMID 27741128.
- ↑ Woo, Seong-Dae; Luu, Quoc Quang; Park, Hae-Sim (28 July 2020). "NSAID-Exacerbated Respiratory Disease (NERD): From Pathogenesis to Improved Care". Frontiers in Pharmacology 11: 1147. doi:10.3389/fphar.2020.01147. ISSN 1663-9812. PMID 32848759.
- ↑ van Geffen, Wouter H.; Tan, Daniel J.; Walters, Julia Ae; Walters, E. Haydn (2023-12-06). "Inhaled corticosteroids with combination inhaled long-acting beta2-agonists and long-acting muscarinic antagonists for chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews 2023 (12): CD011600. doi:10.1002/14651858.CD011600.pub3. ISSN 1469-493X. PMID 38054551.
- ↑ Hickey, A.J., ed (2004). Pharmaceutical Inhalation Aerosol Technology (2nd ed.). NY: Marcel Dekker. ISBN 9780824742539. https://archive.org/details/pharmaceuticalin134anth.
- ↑ 11.0 11.1 11.2 11.3 "Device use errors with soft mist inhalers: A global systematic literature review and meta-analysis". Chronic Respiratory Disease 17: 1479973119901234. January 2020. doi:10.1177/1479973119901234. PMID 31984767.
- ↑ 12.0 12.1 12.2 "Mobile Health and Inhaler-Based Monitoring Devices for Asthma Management". The Journal of Allergy and Clinical Immunology. In Practice 7 (8): 2535–2543. November 2019. doi:10.1016/j.jaip.2019.08.034. PMID 31706485.
- ↑ 13.0 13.1 "Real-life inhaler technique in asthma patients using the electronic ProAir Digihaler". Airway Pharmacology and Treatment (European Respiratory Society): PA4258. 2019-09-28. doi:10.1183/13993003.congress-2019.PA4258.
- ↑ "In vitro evaluation of an asthma dosing device: the smart-inhaler". Respiratory Medicine 100 (5): 841–5. May 2006. doi:10.1016/j.rmed.2005.09.004. PMID 16216485.
- ↑ 15.0 15.1 Nick Baumann (July–August 2011). "Why You're Paying More to Breathe". Mother Jones. http://motherjones.com/environment/2011/07/cost-increase-asthma-inhalers-expensive.
- ↑ 16.0 16.1 "Asthma Group Concerned "Green" Inhalers May Not be as Effective | ksdk.com | St. Louis, MO". ksdk.com. http://www.ksdk.com/news/living_green/story.aspx?storyid=149094&catid=116.
- ↑ "Withdrawal of albuterol inhalers containing chlorofluorocarbon propellants". N. Engl. J. Med. 356 (13): 1344–51. March 2007. doi:10.1056/NEJMra050380. PMID 17392304.
- ↑ Zeinali, Faeze; Mohammad Karimi, Naser; Jafari, Mohamadali; Akbarzadeh Moghadam, Ebrahim (2021). "Rapid and Deep versus Normal Breathing in Salbutamol Inhalation Effectiveness; a Letter to Editor". Archives of Academic Emergency Medicine 9 (1): e42. doi:10.22037/aaem.v9i1.1122. ISSN 2645-4904. PMID 34223187.
- ↑ "How the Lungs Work - How Your Body Controls Breathing | NHLBI, NIH" (in en). 2022-03-24. https://www.nhlbi.nih.gov/health/lungs/body-controls-breathing#:~:text=The%20diaphragm%20is%20the%20main,such%20as%20during%20physical%20activity.
- ↑ "Inhalation Technique Errors with Metered-Dose Inhalers Among Patients with Obstructive Lung Diseases: A Systematic Review and Meta-Analysis of U.S. Studies". Chronic Obstructive Pulmonary Diseases 6 (3): 267–280. July 2019. doi:10.15326/jcopdf.6.3.2018.0168. PMID 31342732.
- ↑ Sanchis, Joaquin; Gich, Ignasi; Pedersen, Soren (1 August 2016). "Systematic Review of Errors in Inhaler Use: Has Patient Technique Improved Over Time?" (in en). Chest 150 (2): 394–406. doi:10.1016/j.chest.2016.03.041. ISSN 0012-3692. PMID 27060726.
- ↑ "School-based self-management interventions for asthma in children and adolescents: a mixed methods systematic review". The Cochrane Database of Systematic Reviews 1 (1): CD011651. January 2019. doi:10.1002/14651858.CD011651.pub2. PMID 30687940.
- ↑ Feldman, William B.; Bloomfield, Doni; Beall, Reed F.; Kesselheim, Aaron S. (May 17, 2022). "Patents And Regulatory Exclusivities On Inhalers For Asthma And COPD, 1986–2020". Health Affairs 41 (6): 787–796. doi:10.1377/hlthaff.2021.01874. PMID 35579925.
- ↑ Cohen, J. Solis (1876). Inhalation in the treatment of disease: its therapeutics and practice. Philadelphia: Lindsay & Blakiston. https://archive.org/stream/63720410R.nlm.nih.gov/63720410R.
- ↑ Bleyer, J. Mount (1890). "A new method of larygeal and bronchial medication by means of a spray and tube during the act of deep inspiration. Read in the Section of Laryngology and Otology at the Forty-first Annual Meeting of the American Medical Association, Nashville, Tenn., May, 1890.". Journal of the American Medical Association 15 (18): 634–636. doi:10.1001/jama.1890.02410440006001a. https://zenodo.org/record/1447247.
- ↑ Wexler RE (1968). Analgizer: Inhaler for supervised self-administration of inhalation anesthesia. Abbott Park, Illinois: Abbott Laboratories. http://www.trademarkia.com/analgizer-72302697.html. Retrieved 2010-11-21.
- ↑ 27.0 27.1 27.2 27.3 "The "analgizer" in a general hospital: a preliminary report". Canadian Journal of Anesthesia 17 (3): 275–8. 1970. doi:10.1007/BF03004607. PMID 5512851.
- ↑ "Methoxyflurane analgesia for burns dressings: experience with the Analgizer (subscription required)". British Journal of Anaesthesia 41 (12): 1080–5. 1969. doi:10.1093/bja/41.12.1080. PMID 4903969.
- ↑ "Methoxyflurane as an obstetric analgesic: a comparison with trichloroethylene". BMJ 2 (5529): 1554–61. 1966. doi:10.1136/bmj.2.5529.1554. PMID 5926260.
- ↑ "Methoxyflurane: preliminary report on analgesic and mood modifying properties in dentistry (subscription required)". Journal of the American Dental Association 75 (5): 1176–81. 1967. doi:10.14219/jada.archive.1967.0358. PMID 5233333.
- ↑ Firn S (1972). "Methoxyflurane analgesia for burns dressings and other painful ward procedures in children (subscription required)". British Journal of Anaesthesia 44 (5): 517–22. doi:10.1093/bja/44.5.517. PMID 5044082.
- ↑ "The Cardiff Inhaler and Penthrane. A method of sedation analgesia in routine dentistry". Journal of the Dental Association of South Africa 29 (2): 77–80. 1974. PMID 4534883.
- ↑ "A pilot study of inhaled methoxyflurane for procedural analgesia in children (subscription required)". Pediatric Anesthesia 17 (2): 148–53. 2007. doi:10.1111/j.1460-9592.2006.02037.x. PMID 17238886.
- ↑ "Efficacy and safety of methoxyflurane analgesia in the emergency department and prehospital setting". Emergency Medicine Australasia 21 (1): 4–11. 2009. doi:10.1111/j.1742-6723.2009.01153.x. PMID 19254307.
- ↑ "Inhaled methoxyflurane as a prehospital analgesic in children (subscription required)". Emergency Medicine Australasia 18 (4): 404–10. 2006. doi:10.1111/j.1742-6723.2006.00874.x. PMID 16842312.
- ↑ McLennan JV (2007). "Is methoxyflurane a suitable battlefield analgesic?". Journal of the Royal Army Medical Corps 153 (2): 111–3. doi:10.1136/jramc-153-02-08. PMID 17896540. http://ramcjournal.com/2007/jun07/mclennan.pdf.
- ↑ Medical Developments International Pty. Ltd. (2009). "PENTHROX (methoxyflurane) Inhalation: Product Information". Springvale, Victoria, Australia: Medical Developments International Limited. http://www.medicaldev.com/pdf_files/Products_Pain_Relief_Healthcare_Professionals_Medical/Product%20Information%20Sheet.pdf.
- ↑ National Prescribing Service (2010). "Methoxyflurane (Penthrox) for analgesia (doctor's bag listing)". NPS RADAR. Canberra, Australia: National Prescribing Service, Department of Health and Ageing. http://www.nps.org.au/__data/assets/pdf_file/0008/87866/OKA7754_NPS_RADAR_Methoxyflurane_V3.pdf.[yes|permanent dead link|dead link}}]
Further reading
- Patton J (February 1998). "Breathing life into protein drugs — Inhalation of therapeutic macromolecules is a feasible, natural, more people-friendly, delivery system". Nat. Biotechnol. 16 (2): 141–3. doi:10.1038/nbt0198-141. PMID 9487516.
External links
- Basics aspects of inhaled pharmaceutical aerosols
- Recent advances in spray medication technology
- Discrete simulation of powder dispersion in pharmaceutical aerosol inhalers
 |
 KSF
KSF