Anticoagulant
Topic: Medicine
 From HandWiki - Reading time: 28 min
From HandWiki - Reading time: 28 min
| Anticoagulant | |
|---|---|
| Drug class | |
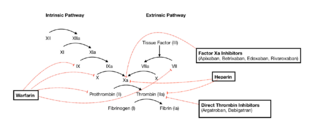 Coagulation cascade and major classes of anticoagulants | |
| Class identifiers | |
| ATC code | B01 |
| External links | |
| MeSH | D00534-class |
An anticoagulant, commonly known as a blood thinner, is a chemical substance that prevents or reduces coagulation of blood, prolonging the clotting time.[1] Some of them occur naturally in blood-eating animals such as leeches and mosquitoes, where they help keep the bite area unclotted long enough for the animal to obtain some blood.[2][3]
As a class of medications, anticoagulants are used in therapy for thrombotic disorders.[4] Oral anticoagulants (OACs) are taken by many people in pill or tablet form, and various intravenous anticoagulant dosage forms are used in hospitals.[5][6] Some anticoagulants are used in medical equipment, such as sample tubes, blood transfusion bags, heart–lung machines, and dialysis equipment.[7][8] One of the first anticoagulants, warfarin, was initially approved as a rodenticide.[9]
Anticoagulants are closely related to antiplatelet drugs and thrombolytic drugs by manipulating the various pathways of blood coagulation.[10] Specifically, antiplatelet drugs inhibit platelet aggregation (clumping together), whereas anticoagulants inhibit specific pathways of the coagulation cascade, which happens after the initial platelet aggregation but before the formation of fibrin and stable aggregated platelet products.[11][12]
Common anticoagulants include warfarin and heparin.[13]
Medical uses
The use of anticoagulants is a decision based upon the risks and benefits of anticoagulation.[14] The biggest risk of anticoagulation therapy is the increased risk of bleeding.[15] In otherwise healthy people, the increased risk of bleeding is minimal, but those who have had recent surgery, cerebral aneurysms, and other conditions may have too great a risk of bleeding.[16][17] Generally, the benefit of anticoagulation is prevention of or reduction of progression of a thromboembolic disease.[18] Some indications for anticoagulant therapy that are known to have benefit from therapy include:
- Atrial fibrillation — commonly forms an atrial appendage clot[19]
- Coronary artery disease[20]
- Deep vein thrombosis — can lead to pulmonary embolism[21]
- Ischemic stroke[22]
- Hypercoagulable states (e.g., Factor V Leiden) — can lead to deep vein thrombosis[23]
- Mechanical heart valves[24]
- Myocardial infarction[25]
- Pulmonary embolism[26]
- Restenosis from stents[27]
- Cardiopulmonary bypass (or any other surgeries requiring temporary aortic occlusion)[28]
- Heart failure[29]
In these cases, anticoagulation therapy can prevent formation of dangerous clots or prevent growth of clots.[30]
The decision to begin therapeutic anticoagulation often involves the use of multiple bleeding risk predictable outcome tools as non-invasive pre-test stratifications due to the potential for bleeds while on blood thinning agents.[15] Among these tools are HAS-BLED,[31] ATRIA,[32] HEMORR2HAGES,[33] and CHA2DS2-VASc.[34] The risk of bleeding using the aforementioned risk assessment tools must then be weighed against thrombotic risk in order to formally determine patient's overall benefit in starting anticoagulation therapy.[35]
There is no evidence to indicate that adding anticoagulant therapy to standard treatment has a benefit for people with cerebral small vessel disease but not dementia and there is an increased risk of a person with this disease experiencing a bleed with this approach.[36]
Adverse effects
The most serious and common adverse side effect associated with anticoagulant are increased risk of bleeding, both nonmajor and major bleeding events.[37] Risk of bleeding is dependent on the class of anticoagulant agent used, patient's age, and pre-existing health conditions. Warfarin has an estimated incidence of bleeding of 15-20% per year and life-threatening bleeding rate of 1-3% per year.[38] Newer non-vitamin K antagonist oral anticoagulants appear to have fewer life-threatening bleeding events compared to warfarin.[39][40] Additionally, patients aged 80 years or more may be especially susceptible to bleeding complications, with a rate of 13 bleeds per 100 person-years.[41] Bleeding risk is especially important to consider in patients with renal impairment and NOAC therapy due to the fact that all NOACs, to some extent, are excreted by the kidneys.[42] Thus, patients with renal impairment may be at higher risk of increased bleeding.[43]
In people with cancer, a systematic review has found warfarin had no effect on death rate or the risk of blood clots.[44] However it did increase the risk of major bleeding in 107 more people per 1000 population and minor bleeding in 167 more people in 1000 population.[44] Apixaban had no effect on mortality, recurrence of blood clots in blood vessels or major bleeding or minor bleeding, however this finding comes only from one study.[44]
Nonhemorrhagic adverse events are less common than hemorrhagic adverse events but should still be monitored closely.[39] Nonhemorrhagic adverse events of warfarin include skin necrosis, limb gangrene, and purple toe syndrome.[45] Skin necrosis and limb gangrene are most commonly observed on the third to eighth day of therapy.[46][47] The exact pathogenesis of skin necrosis and limb gangrene are not completely understood but are believed to be associated with warfarin's effect on inhibiting production of protein C and protein S.[48][49] Purple toe syndrome typically develops three to eight weeks after initiation of warfarin therapy.[50][51] Other adverse effects of warfarin are associated with depletion of vitamin K, which can lead to inhibition of G1a proteins and growth arrest-specific gene 6, which can lead to increased risk of arterial calcification and heart valve, especially if too much Vitamin D is present.[52][53] Warfarin's interference of G1a proteins have also been linked to abnormalities in fetal bone development in mothers who were treated with warfarin during pregnancy.[54][55] Long-term warfarin and heparin usage have also been linked to osteoporosis.[56][45]
Another potentially serious complication associated with heparin use is called heparin-induced thrombocytopenia (HIT).[57] There are two distinct types of HIT 1) immune-mediated and 2) non-immune mediated.[57] Immune-mediated HIT most commonly arises five to ten days after exposure to heparin.[58] Pathogenesis of immune-mediated HIT is believed to be caused by heparin-dependent immunoglobulin antibodies binding to platelet factor 4/heparin complexes on platelets, leading to wide spread platelet activation.[59]
Interactions
Foods and food supplements with blood-thinning effects include nattokinase, lumbrokinase, beer, bilberry, celery, cranberries, fish oil, garlic, ginger, ginkgo, ginseng, green tea, horse chestnut, licorice, niacin, onion, papaya, pomegranate, red clover, soybean, St. John's wort, turmeric, wheatgrass, and willow bark.[60][61][62] Many herbal supplements have blood-thinning properties, such as danshen and feverfew.[63] Multivitamins that do not interact with clotting are available for patients on anticoagulants.[64]
However, some foods and supplements encourage clotting.[65] These include alfalfa, avocado, cat's claw, coenzyme Q10, and dark leafy greens such as spinach.[66][67] Excessive intake of aforementioned food should be avoided whilst taking anticoagulants or, if coagulability is being monitored, their intake should be kept approximately constant so that anticoagulant dosage can be maintained at a level high enough to counteract this effect without fluctuations in coagulability.[68][69]
Grapefruit interferes with some anticoagulant drugs, increasing the amount of time it takes for them to be metabolized out of the body, and so should be eaten with caution when on anticoagulant drugs.[70]
Anticoagulants are often used to treat acute deep vein thrombosis.[71][72] People using anticoagulants to treat this condition should avoid using bed rest as a complementary treatment because there are clinical benefits to continuing to walk and remaining mobile while using anticoagulants in this way.[73] Bed rest while using anticoagulants can harm patients in circumstances in which it is not medically necessary.[73]
Types
A number of anticoagulants are available. Warfarin, other coumarins, and heparins have long been used.[74] Since the 2000s, a number of agents have been introduced that are collectively referred to as direct oral anticoagulants (DOACs), previously named novel oral anticoagulants (NOACs) or non-vitamin K antagonist oral anticoagulants.[75][76][77][78] These agents include direct thrombin inhibitor (dabigatran) and factor Xa inhibitor (rivaroxaban, apixaban, betrixaban and edoxaban) and they have been shown to be as good or possibly better than the coumarins with less serious side effects.[79] The newer anticoagulants (NOACs/DOACs), are more expensive than the traditional ones and should be used with care in patients with kidney problems.[80]
Coumarins (vitamin K antagonists)
These oral anticoagulants are derived from coumarin, which is found in many plants. A prominent member of this class, warfarin (Coumadin), was found to be the anticoagulant most prescribed in a large multispecialty practice.[81] It takes at least 48 to 72 hours for the anticoagulant effect to develop. Where an immediate effect is required, heparin is given concomitantly. These anticoagulants are used to treat patients with deep-vein thrombosis (DVT), pulmonary embolism (PE) and to prevent emboli in patients with atrial fibrillation (AF), and mechanical prosthetic heart valves. Other examples are acenocoumarol, phenprocoumon, atromentin, and phenindione.[citation needed]
The coumarins brodifacoum and difenacoum are used as mammalicides (particularly as rodenticides), but are not used medically.[citation needed]
Heparin and derivative substances
Heparin is the most widely used intravenous clinical anticoagulant worldwide.[82] Heparin is a naturally occurring glycosaminoglycan. There are three major categories of heparin: unfractionated heparin (UFH), low molecular weight heparin (LMWH), and ultra-low-molecular weight heparin (ULMWH).[83] Unfractionated heparin is usually derived from pig intestines and bovine lungs.[84] UFH binds to the enzyme inhibitor antithrombin III (AT), causing a conformational change that results in its activation.[85] The activated AT then inactivates factor Xa, thrombin, and other coagulation factors.[86] Heparin can be used in vivo (by injection), and also in vitro to prevent blood or plasma clotting in or on medical devices. In venipuncture, Vacutainer brand blood collecting tubes containing heparin usually have a green cap.[citation needed]
Low molecular weight heparin (LMWH)
Low molecular weight heparin (LMWH), is produced through a controlled depolymerization of unfractionated heparin.[83] LMWH exhibits higher anti-Xa/anti-IIa activity ratio and is useful as it does not require monitoring of the APTT coagulation parameter and has fewer side effects.[83]
Synthetic pentasaccharide inhibitors of factor Xa
- Fondaparinux is a synthetic sugar composed of the five sugars (pentasaccharide) in heparin that bind to antithrombin. It is a smaller molecule than low molecular weight heparin.
- Idraparinux
- Idrabiotaparinux
Direct oral
The direct oral anticoagulants (DOACs) were introduced in and after 2008.[87] There are five DOACs currently on the market: dabigatran, rivaroxaban, apixaban, edoxaban and betrixaban.[88] They were also previously referred to as "new/novel" and "non-vitamin K antagonist" oral anticoagulants (NOACs).[89]
Compared to warfarin, DOACs have a rapid onset action and relatively short half-lives; hence, they carry out their function more rapidly and effectively and allow for drugs to quickly reduce their anticoagulation effects.[90] Routine monitoring and dose adjustments of DOACs is less important than for warfarin, as they have better predictable anticoagulation activity.[91] DOAC monitoring, including laboratory monitoring and a complete medication review, should generally be conducted before initiation of a DOAC, 1–3 months after initiation, and then every 6–12 months afterwards.[92]
Both DOACs and warfarin are equivalently effective but compared to warfarin, DOACs have fewer drug interactions, no known dietary interactions, wider therapeutic index, and have conventional dosing that does not require dose adjustments with constant monitoring.[93][91] However, there is presently no countermeasure for most DOACs, unlike for warfarin; nonetheless, the short half-lives of DOACs will allow their effects to swiftly recede. A reversal agent for dabigatran, idarucizumab, is currently available and approved for use by the FDA. Rates of adherence to DOACs are only modestly higher than adherence to warfarin among patients prescribed these drugs, and thus adherence to anticoagulation is often poor, despite hopes that DOACs would lead to higher adherence rates.[94]
DOACs are significantly more expensive than warfarin, but the patients on DOACs may experience reduced lab costs, as they do not need to monitor their INR.[92]
Direct factor Xa inhibitors
Drugs such as rivaroxaban, apixaban and edoxaban work by inhibiting factor Xa directly (unlike the heparins and fondaparinux, which work via antithrombin activation). Also included in this category are betrixaban from Portola Pharmaceuticals, the discontinued darexaban (YM150) from Astellas, and, more recently, the discontinued letaxaban (TAK-442) from Takeda and eribaxaban (PD0348292) from Pfizer. Betrixaban is significant as it was, in 2018, the only oral factor Xa inhibitor approved by the FDA for use in acutely medically ill patients.[95] Darexaban development was discontinued in September 2011; in a trial for prevention of recurrences of myocardial infarction in addition to dual antiplatelet therapy (DAPT), the drug did not demonstrate effectiveness, and the risk of bleeding was increased by approximately 300%.[96] The development of letaxaban was discontinued for acute coronary syndrome in May 2011, following negative results from a Phase II study.[97]
Direct thrombin inhibitors
Another type of anticoagulant is the direct thrombin inhibitor.[98] Current members of this class include the bivalent drugs hirudin, lepirudin, and bivalirudin; and the monovalent drugs argatroban and dabigatran. An oral direct thrombin inhibitor, ximelagatran (Exanta) was denied approval by the Food and Drug Administration (FDA) in September 2004[99] and was pulled from the market entirely in February 2006 after reports of severe liver damage and heart attacks.[100] In November 2010, dabigatran etexilate was approved by the FDA to prevent thrombosis in atrial fibrillation.
Relevance to dental treatments
As in any invasive procedures, patients on anticoagulation therapy have increased risk for bleeding and caution should be used along with local hemostatic methods to minimize bleeding risk during the operation as well as post-operatively.[101] However, with regards to DOACs and invasive dental treatments, there has not been enough clinical evidence and experience to prove any reliable adverse effects, relevance or interaction between these two.[102] Further clinical prospective studies on DOACs are required to investigate the bleeding risk and haemostasis associated to surgical dental procedures.[103]
Recommendations of modifications to usage/dosage of DOACs prior to dental treatments are made based on the balance of the bleeding risk of each procedure and also the individual's bleeding own bleeding risks and renal functionality.[104] With low-bleeding-risk dental procedures, it is recommended that DOACs be continued by the patient, so as to avoid any increase in the risk of a thromboembolic event.[105][106] For dental procedures with a higher risk of bleeding complications (i.e. complex extractions, adjacent extractions leading to a large wound, or more than three extractions), the recommended practice is for the patient to miss or delay a dose of their DOAC before such procedures so as to minimize the effect on bleeding risk.[107]
Antithrombin protein therapeutics
The antithrombin protein itself is used as a protein therapeutic that can be purified from human plasma[108] or produced recombinantly (for example, Atryn, which is produced in the milk of genetically modified goats).[109][110]
Antithrombin is approved by the FDA as an anticoagulant for the prevention of clots before, during, or after surgery or birthing in patients with hereditary antithrombin deficiency.[108][110]
Other
Many other anticoagulants exist, for use in research and development, diagnostics, or as drug candidates.
- Batroxobin, a toxin from a snake venom, clots platelet-rich plasma without affecting platelet functions (cleaves fibrinogen).
- Hementin is an anticoagulant protease from the salivary glands of the giant Amazon leech, Haementeria ghilianii.
- Vitamin E
- Alcoholic beverage
Reversal agents
With the growing number of patients taking oral anticoagulation therapy, studies into reversal agents are gaining increasing interest due to major bleeding events and need for urgent anticoagulant reversal therapy.[111] Reversal agents for warfarin are more widely studied and established guidelines for reversal exist, due to longer history of use of warfarin and the ability to get a more accurate measurement of anticoagulation effect in a patient via measuring the INR (International Normalized Ratio).[112] In general, vitamin K is most commonly used in order to reverse the effect of warfarin in non-urgent settings.[113] However, in urgent settings, or in settings with extremely high INR (INR >20), hemostatic reversal agents such as fresh frozen plasma (FFP), recombinant factor VIIa, and prothrombin complex concentrate (PCC) have been utilized with proven efficacy.[114] Specifically with warfarin, four factor PCC (4F-PCC) has been shown to have superior safety and mortality benefits compared to FPP in lowering INR levels.[111]
Although specific antidotes and reversal agents for DOACs are not as widely studied, idarucizumab (for dabigatran) and andexanet alfa (for factor Xa inhibitor) have been used in clinical settings with varying efficacy.[89] Idarucizumab is a monoclonal antibody, approved by the US FDA in 2015, that reverses effect of dabigatran by binding to both free and thrombin-bound dabigatran.[115][116] Andexanet alfa is a recombinant modified human factor Xa decoy that reverses the effect of factor Xa inhibitors by binding at the active sites of factor Xa inhibitor and making it catalytically inactive.[117][118] Andexanet alfa was approved by US FDA in 2018.[119] Another drug called ciraparantag, a potential reversal agent for direct factor Xa inhibitors, is still under investigation.[120] Additionally, hemostatic reversal agents have also been used with varying efficacy to reverse effects of DOACs.[121][122]
Coagulation inhibitor measurement
A Bethesda unit (BU) is a measure of blood coagulation inhibitor activity. It is the amount of inhibitor that will inactivate half of a coagulant during the incubation period.[123] It is the standard measure used in the United States, and is so named because it was adopted as a standard at a conference in Bethesda, Maryland.[124]
Laboratory use
Laboratory instruments, blood transfusion bags, and medical and surgical equipment will get clogged up and become non-operational if blood is allowed to clot. In addition, test tubes used for laboratory blood tests will have chemicals added to stop blood clotting. Apart from heparin, most of these chemicals work by binding calcium ions, preventing the coagulation proteins from using them.
- Ethylenediaminetetraacetic acid (EDTA) strongly and irreversibly chelates (binds) calcium ions, preventing blood from clotting.
- Citrate is in liquid form in the tube and is used for coagulation tests, as well as in blood transfusion bags. It binds the calcium, but not as strongly as EDTA. Correct proportion of this anticoagulant to blood is crucial because of the dilution, and it can be reversed with the addition of calcium. Formulations include plain sodium citrate, acid-citrate-dextrose, and more.
- Oxalate has a mechanism similar to that of citrate. It is the anticoagulant used in fluoride/oxalate tubes used to determine glucose and lactate levels. The fluoride serves to inhibit glycolysis, which can throw off blood sugar measurements. In fact, citrate/fluoride/EDTA tubes work better in this regard.[125]
Dental considerations for long-term users
Dental practitioners play an important role in the early detection of anticoagulant overdose through oral manifestations as the patient does not show any symptoms. Dental treatment of patients taking anticoagulant or antiplatelet medication raises safety concerns in terms of the potential risk of bleeding complications following invasive dental procedures. Therefore, there comes the need for certain guidelines for the dental care of patients taking these drugs.
Detecting overdose
An overdose in anticoagulants usually occurs in people who have heart problems and need to take anticoagulants in a long term, in order to reduce the risk of stroke from their high blood pressure.
An International Normalised Ratio (INR) test would be recommended, to confirm the overdose so that the dosage can be adjusted to an acceptable standard. The INR test measures the time taken for a clot to form in a blood sample, relative to a standard.
An INR value of 1 indicates a level of coagulation equivalent to that of an average patient not taking warfarin and values greater than 1 indicate a longer clotting time and thus a longer bleeding time.
Assessing bleeding risk
There are 2 main parts to the assessment of bleeding risk:
- Assessment of the likely risk of bleeding associated with the required dental procedure
- Assessment of the patient's individual level bleeding risk
Managing bleeding risk
A patient who is on anticoagulants or antiplatelet medications may undergo dental treatments which are unlikely to cause bleeding such as local anaesthesia injection, basic gum charting, removal of plaque, calculus and stain above the gum level, direct or indirect fillings which are above the gingiva, root canal treatment, taking impression for denture or crown and fitting or adjustment of orthodontic appliances. For all these procedures, it is recommended for the dentist to treat the patient following the normal standard procedure and taking care to avoid any bleeding.
For a patient who needs to undergo dental treatments which is more likely to cause bleeding such as simple tooth extractions (1-3 teeth with small wound size), drainage of swelling inside the mouth, periodontal charting, root planing, direct or indirect filling which extends below the gingiva, complex filling, flap raising procedure, gingival recontouring and biopsies, the dentist needs to take extra precautions apart from the standard procedure. The recommendations[126] are as follows:
- if the patient has another medical condition or taking other medication that may increase bleeding risk, consult the patient's general medical practitioner or specialist
- if the patient is on a short course anticoagulant or antiplatelet therapy, delay non-urgent, invasive procedure, until the medication has been discontinued
- plan treatment for early in the day and week, where possible, to allow time for the management of prolonged bleeding or re-bleeding, if it occurs
- perform the procedure as atraumatically as possible, use appropriate local measures and only discharge patient once haemostasis has been confirmed
- if travel time to emergency care is a concern, place particular emphasis at the time of the initial treatment on the use of measures to avoid complications
- advise the patient to take paracetamol, unless contraindicated, for pain relief rather than NSAIDs such as aspirin, ibuprofen, diclofenac or naproxen
- provide the patient with written post-treatment advice and emergency contact details
- follow the specific recommendations and advice given for the management of patients taking the different anticoagulants or antiplatelet drugs
There is general agreement that in most cases, treatment regimens with older anticoagulants (e.g., warfarin) and antiplatelet agents (e.g., clopidogrel, ticlopidine, prasugrel, ticagrelor, and/or aspirin) should not be altered before dental procedures. The risks of stopping or reducing these medication regimens (i.e., thromboembolism, stroke, myocardial infarction) far outweigh the consequences of prolonged bleeding, which can be controlled with local measures. In patients with other existing medical conditions that can increase the risk of prolonged bleeding after dental treatment or who are receiving other therapy that can increase bleeding risk, dental practitioners may wish to consult the patient's physician to determine whether care can safely be delivered in a primary care office. Any suggested modification to the medication regimen prior to dental surgery should be done in consultation and on advice of the patient's physician.
On the basis of limited evidence, general consensus appears to be that in most patients who are receiving the newer direct-acting oral anticoagulants (i.e., dabigatran, rivaroxaban, apixaban, or edoxaban) and undergoing dental treatment (in conjunction with usual local measures to control bleeding), no change to the anticoagulant regimen is required. In patients deemed to be at higher risk of bleeding (e.g., patients with other medical conditions or undergoing more extensive procedures associated with higher bleeding risk), consideration may be given, in consultation with and on advice of the patient's physician, to postponing the timing of the daily dose of the anticoagulant until after the procedure; timing the dental intervention as late as possible after last dose of anticoagulant; or temporarily interrupting drug therapy for 24 to 48 hours.
Research
A substantial number of compounds is being investigated for use as anticoagulants. The most promising ones act on the contact activation system (factor XIIa and factor XIa); it is anticipated that this may provide agents that prevent thrombosis without conferring a risk of bleeding.[127]
As of November 2021[update], the direct factor XIa inhibitor milvexian is in Phase II clinical trials for the prevention of embolism after surgery.[128]
See also
- Hypercoagulability in pregnancy
- CHADS2 score
- Direct factor Xa inhibitors
References
- ↑ "Overview: Anticoagulant medicines". NHS. 26 July 2021. https://www.nhs.uk/conditions/anticoagulants/.
- ↑ "Chemical and mechanical alternatives to leech therapy: a systematic review and critical appraisal". Journal of Reconstructive Microsurgery (Thieme Medical Publishers) 27 (8): 481–6. October 2011. doi:10.1055/s-0031-1284233. PMID 21780018. https://www.thieme-connect.de/products/ejournals/abstract/10.1055/s-0031-1284233.
- ↑ "Detection of heparin in the salivary gland and midgut of Aedes togoi". The Korean Journal of Parasitology (The Korean Society for Parasitology and Tropical Medicine) 52 (2): 183–8. April 2014. doi:10.3347/kjp.2014.52.2.183. PMID 24850962.
- ↑ "Anticoagulant treatment for subsegmental pulmonary embolism". The Cochrane Database of Systematic Reviews 2020 (2): CD010222. 7 February 2020. doi:10.1002/14651858.CD010222.pub4. ISSN 1469-493X. PMID 32030721.
- ↑ ten Cate, Hugo, ed (2015-12-30). "Comparison of the Novel Oral Anticoagulants Apixaban, Dabigatran, Edoxaban, and Rivaroxaban in the Initial and Long-Term Treatment and Prevention of Venous Thromboembolism: Systematic Review and Network Meta-Analysis". PLOS One 10 (12): e0144856. doi:10.1371/journal.pone.0144856. PMID 26716830. Bibcode: 2015PLoSO..1044856C.
- ↑ "Effectiveness and Safety of Non-vitamin K Antagonist Oral Anticoagulants for Atrial Fibrillation and Venous Thromboembolism: A Systematic Review and Meta-analyses". Clinical Therapeutics 39 (7): 1456–1478.e36. July 2017. doi:10.1016/j.clinthera.2017.05.358. PMID 28668628. https://www.clinicaltherapeutics.com/article/S0149-2918(17)30699-9/fulltext.
- ↑ "The role of ethylenediamine tetraacetic acid (EDTA) as in vitro anticoagulant for diagnostic purposes". Clinical Chemistry and Laboratory Medicine 45 (5): 565–76. 2007-01-01. doi:10.1515/CCLM.2007.110. PMID 17484616. https://www.degruyter.com/document/doi/10.1515/CCLM.2007.110/.
- ↑ "Safe anticoagulation when heart and lungs are "on vacation"". Annals of Translational Medicine 3 (Suppl 1): S11. May 2015. doi:10.3978/j.issn.2305-5839.2015.02.03. PMID 26046056.
- ↑ "Warfarin: almost 60 years old and still causing problems". British Journal of Clinical Pharmacology 62 (5): 509–11. November 2006. doi:10.1111/j.1365-2125.2006.02806.x. PMID 17061959.
- ↑ Patel, S.; Singh, R.; Preuss, C. V.; Patel, N. (2020). "Warfarin". StatPearls (StatPearls Publishing). PMID 29261922. http://www.ncbi.nlm.nih.gov/books/NBK470313/. Retrieved 2020-01-23.
- ↑ "Antiplatelet Medications". StatPearls. StatPearls Publishing. 2020. http://www.ncbi.nlm.nih.gov/books/NBK537062/. Retrieved 2020-01-23.
- ↑ "Anticoagulation drug therapy: a review". The Western Journal of Emergency Medicine 16 (1): 11–7. January 2015. doi:10.5811/westjem.2014.12.22933. PMID 25671002.
- ↑ Winslow, Ron; Johnson, Avery (2007-12-10). "Race Is on for the Next Blood Thinner". The Wall Street Journal: p. A12. https://www.wsj.com/articles/SB119725064671318856. "...in a market now dominated by one of the oldest mainstay pills in medicine: the blood thinner warfarin. At least five next-generation blood thinners are in advanced testing to treat or prevent potentially debilitating or life-threatening blood clots in surgery and heart patients. First candidates could reach the market in 2009."
- ↑ "An Update on the "Novel" and Direct Oral Anticoagulants, and Long-Term Anticoagulant Therapy". Clinics in Chest Medicine 39 (3): 583–593. September 2018. doi:10.1016/j.ccm.2018.04.010. PMID 30122182.
- ↑ 15.0 15.1 "Scoring Systems for Estimating the Risk of Anticoagulant-Associated Bleeding". Seminars in Thrombosis and Hemostasis 43 (5): 514–524. July 2017. doi:10.1055/s-0037-1598061. PMID 28359135.
- ↑ "The hemorrhage risk of prophylactic external ventricular drain insertion in aneurysmal subarachnoid hemorrhage patients requiring endovascular aneurysm treatment: a systematic review and meta-analysis". Journal of Neurosurgical Sciences 61 (1): 53–63. February 2017. doi:10.23736/S0390-5616.16.03244-6. PMID 25963956. http://www.minervamedica.it/index2.php?show=R38Y2017N01A0053.
- ↑ "Meta-Analysis of Usefulness of Anticoagulation After Transcatheter Aortic Valve Implantation". The American Journal of Cardiology 120 (9): 1612–1617. November 2017. doi:10.1016/j.amjcard.2017.07.059. PMID 28844512.
- ↑ "Blood Thinners". https://medlineplus.gov/bloodthinners.html.
- ↑ "Efficacy and Harms of Direct Oral Anticoagulants in the Elderly for Stroke Prevention in Atrial Fibrillation and Secondary Prevention of Venous Thromboembolism: Systematic Review and Meta-Analysis". Circulation 132 (3): 194–204. July 2015. doi:10.1161/CIRCULATIONAHA.114.013267. PMID 25995317.
- ↑ "Antithrombotic therapy in patients with atrial fibrillation and coronary artery disease". Avicenna Journal of Medicine 9 (4): 123–128. 2019. doi:10.4103/ajm.AJM_73_19. PMID 31903386.
- ↑ "Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report". Chest 149 (2): 315–352. February 2016. doi:10.1016/j.chest.2015.11.026. PMID 26867832.
- ↑ "Antiplatelet and Anticoagulant Therapies for Prevention of Ischemic Stroke". Clinical and Applied Thrombosis/Hemostasis 23 (4): 301–318. May 2017. doi:10.1177/1076029616660762. PMID 27461564.
- ↑ "The use of direct oral anticoagulants in inherited thrombophilia". Journal of Thrombosis and Thrombolysis 43 (1): 24–30. January 2017. doi:10.1007/s11239-016-1428-2. PMID 27734187.
- ↑ "Mechanical prosthetic heart valves: Quality of anticoagulation and thromboembolic risk. The observational multicenter PLECTRUM study". International Journal of Cardiology 267: 68–73. September 2018. doi:10.1016/j.ijcard.2018.04.042. PMID 29957264.
- ↑ "Antiplatelet and anticoagulant use after myocardial infarction". Clinical Cardiology 19 (5): 357–65. May 1996. doi:10.1002/clc.4960190506. PMID 8723593.
- ↑ "Management of Pulmonary Embolism: An Update". Journal of the American College of Cardiology 67 (8): 976–90. March 2016. doi:10.1016/j.jacc.2015.11.061. PMID 26916489.
- ↑ "Anticoagulation after coronary stenting: a systemic review". British Medical Bulletin 123 (1): 79–89. September 2017. doi:10.1093/bmb/ldx018. PMID 28910988.
- ↑ "Anticoagulation management during cross-clamping and bypass". Best Practice & Research. Clinical Anaesthesiology 30 (3): 359–70. September 2016. doi:10.1016/j.bpa.2016.07.002. PMID 27650345.
- ↑ Thomas, Isac; EncisoSilva, Jorge; Schlueter, Michelle; Greenberg, Barry (2016), Bauersachs, Johann; Butler, Javed; Sandner, Peter, eds., "Anticoagulation Therapy and NOACs in Heart Failure", Heart Failure (Springer International Publishing) 243: pp. 515–535, doi:10.1007/164_2016_126, ISBN 978-3-319-59658-7, PMID 28233177
- ↑ "Emerging therapeutic uses of direct-acting oral anticoagulants: An evidence-based perspective". Pharmacological Research 120: 206–218. June 2017. doi:10.1016/j.phrs.2017.03.026. PMID 28366835.
- ↑ "HAS-BLED Score for Major Bleeding risk". MDCalc. http://www.mdcalc.com/has-bled-score-for-major-bleeding-risk/.
- ↑ "ATRIA Bleeding Risk". MDCalc. http://www.mdcalc.com/atria-bleeding-risk-score/.
- ↑ "HEMORR₂HAGES Score for Major Bleeding Risk" (in en). https://www.mdcalc.com/hemorr2hages-score-major-bleeding-risk.
- ↑ "CHA2DS2-VASc". MDCalc. http://www.mdcalc.com/cha2ds2-vasc-score-for-atrial-fibrillation-stroke-risk/.
- ↑ "The HAS-BLED Score for Predicting Major Bleeding Risk in Anticoagulated Patients With Atrial Fibrillation: A Systematic Review and Meta-analysis". Clinical Cardiology 38 (9): 555–61. September 2015. doi:10.1002/clc.22435. PMID 26418409.
- ↑ Kwan, Joseph; Hafdi, Melanie; Chiang, Lorraine L W; Myint, Phyo K; Wong, Li Siang; Quinn, Terry J (2022-07-13). Cochrane Dementia and Cognitive Improvement Group. ed. "Antithrombotic therapy to prevent cognitive decline in people with small vessel disease on neuroimaging but without dementia" (in en). Cochrane Database of Systematic Reviews 2022 (7): CD012269. doi:10.1002/14651858.CD012269.pub2. PMID 35833913.
- ↑ "Emergency Reversal of Anticoagulation". The Western Journal of Emergency Medicine 20 (5): 770–783. August 2019. doi:10.5811/westjem.2018.5.38235. PMID 31539334.
- ↑ "Reversal of warfarin-induced hemorrhage in the emergency department". The Western Journal of Emergency Medicine 12 (4): 386–92. November 2011. doi:10.5811/westjem.2011.3.2051. PMID 22224125.
- ↑ 39.0 39.1 "Breadth of complications of long-term oral anticoagulant care". Hematology. American Society of Hematology. Education Program 2018 (1): 432–438. November 2018. doi:10.1182/asheducation-2018.1.432. PMID 30504343.
- ↑ "Safety and effectiveness of oral rivaroxaban versus standard anticoagulation for the treatment of symptomatic deep-vein thrombosis (XALIA): an international, prospective, non-interventional study". The Lancet. Haematology 3 (1): e12-21. January 2016. doi:10.1016/S2352-3026(15)00257-4. PMID 26765643.
- ↑ "Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation". Circulation 115 (21): 2689–96. May 2007. doi:10.1161/CIRCULATIONAHA.106.653048. PMID 17515465.
- ↑ "Nonvitamin K antagonist oral anticoagulant use in patients with renal impairment". Therapeutic Advances in Cardiovascular Disease 11 (9): 243–256. September 2017. doi:10.1177/1753944717714921. PMID 28651452.
- ↑ "Influence of Renal Function on the Pharmacokinetics, Pharmacodynamics, Efficacy, and Safety of Non-Vitamin K Antagonist Oral Anticoagulants". Mayo Clinic Proceedings 93 (10): 1503–1519. October 2018. doi:10.1016/j.mayocp.2018.06.018. PMID 30286834.
- ↑ 44.0 44.1 44.2 Kahale, Lara A; Hakoum, Maram B; Tsolakian, Ibrahim G; Matar, Charbel F; Barba, Maddalena; Yosuico, Victor ED; Terrenato, Irene; Sperati, Francesca et al. (2017-12-29). "Oral anticoagulation in people with cancer who have no therapeutic or prophylactic indication for anticoagulation". Cochrane Database of Systematic Reviews 12 (12): CD006466. doi:10.1002/14651858.cd006466.pub6. ISSN 1465-1858. PMID 29285754.
- ↑ 45.0 45.1 "Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines". Chest 141 (2 Suppl): e44S–e88S. February 2012. doi:10.1378/chest.11-2292. PMID 22315269.
- ↑ "Local haemorrhage and necrosis of the skin and underlying tissues, during anti-coagulant therapy with dicumarol or dicumacyl". Acta Medica Scandinavica 148 (6): 453–67. 2009-04-24. doi:10.1111/j.0954-6820.1954.tb01741.x. PMID 13171021.
- ↑ "Warfarin necrosis of the skin and subcutaneous tissue of the male external genitalia". The Journal of Urology 130 (2): 352–4. August 1983. doi:10.1016/S0022-5347(17)51147-7. PMID 6876290.
- ↑ "Protein C and the development of skin necrosis during anticoagulant therapy". Thrombosis and Haemostasis 49 (3): 251. June 1983. doi:10.1055/s-0038-1657378. PMID 6688309.
- ↑ "Necrosis of skin induced by coumarin in a patient deficient in protein S". BMJ 298 (6668): 233–4. January 1989. doi:10.1136/bmj.298.6668.233. PMID 2522326.
- ↑ "Purple toes syndrome associated with warfarin therapy in a patient with antiphospholipid syndrome". Pharmacotherapy 23 (5): 674–7. May 2003. doi:10.1592/phco.23.5.674.32200. PMID 12741443.
- ↑ "Purple toe syndrome following anticoagulant therapy". British Journal of Haematology 114 (4): 740. September 2001. doi:10.1046/j.1365-2141.2001.03107.x. PMID 11564060.
- ↑ "Vitamin K in the treatment and prevention of osteoporosis and arterial calcification". American Journal of Health-System Pharmacy 62 (15): 1574–81. August 2005. doi:10.2146/ajhp040357. PMID 16030366. http://www.scicompdf.se/osteolis/adams_2005.pdf.
- ↑ "Vitamin K-dependent proteins, warfarin, and vascular calcification". Clinical Journal of the American Society of Nephrology 3 (5): 1504–10. September 2008. doi:10.2215/CJN.00770208. PMID 18495950.
- ↑ "Congenital malformations associated with the administration of oral anticoagulants during pregnancy". The Journal of Pediatrics 86 (3): 459–62. March 1975. doi:10.1016/S0022-3476(75)80986-3. PMID 1113236.
- ↑ "Maternal and fetal sequelae of anticoagulation during pregnancy". The American Journal of Medicine 68 (1): 122–40. January 1980. doi:10.1016/0002-9343(80)90181-3. PMID 6985765.
- ↑ "Risk of osteoporotic fracture in elderly patients taking warfarin: results from the National Registry of Atrial Fibrillation 2". Archives of Internal Medicine 166 (2): 241–6. January 2006. doi:10.1001/archinte.166.2.241. PMID 16432096.
- ↑ 57.0 57.1 "Heparin-induced thrombocytopenia". Circulation 114 (8): e355-6. August 2006. doi:10.1161/CIRCULATIONAHA.106.632653. PMID 16923760.
- ↑ "Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition)". Chest 133 (6 Suppl): 340S–380S. June 2008. doi:10.1378/chest.08-0677. PMID 18574270.
- ↑ "Systematic review of fondaparinux for heparin-induced thrombocytopenia: When there are no randomized controlled trials". Research and Practice in Thrombosis and Haemostasis 2 (4): 678–683. October 2018. doi:10.1002/rth2.12145. PMID 30349886.
- ↑ "Drug interactions update: drugs, herbs, and oral anticoagulation". Journal of Thrombosis and Thrombolysis 12 (1): 67–71. September 2001. doi:10.1023/A:1012742628628. PMID 11711691.
- ↑ Rui, Tian-Qi; Zhang, Liang; Qiao, Hong-Zhi; Huang, Ping; Qian, Shuai; Li, Jun-Song; Chen, Zhi-Peng; Fu, Ting-Ming et al. (January 2016). "Preparation and Physicochemical and Pharmacokinetic Characterization of Ginkgo Lactone Nanosuspensions for Antiplatelet Aggregation" (in en). Journal of Pharmaceutical Sciences 105 (1): 242–249. doi:10.1016/j.xphs.2015.10.002. PMID 26852855.
- ↑ Yun, Yeo-Pyo; Do, Jae-Ho; Ko, Sung-Ryong; Ryu, Shi-Yong; Kim, Jung-Hyo; Song, Ho-Cheol; Park, Young-Doo; Ahn, Kyoo-Seok et al. (October 2001). "Effects of Korean red ginseng and its mixed prescription on the high molecular weight dextran-induced blood stasis in rats and human platelet aggregation" (in en). Journal of Ethnopharmacology 77 (2–3): 259–264. doi:10.1016/S0378-8741(01)00303-8. PMID 11535373.
- ↑ Choi, Songie; Oh, Dal-Seok; Jerng, Ui Min (2017-08-10). Borrelli, Francesca. ed. "A systematic review of the pharmacokinetic and pharmacodynamic interactions of herbal medicine with warfarin" (in en). PLOS ONE 12 (8): e0182794. doi:10.1371/journal.pone.0182794. ISSN 1932-6203. PMID 28797065. Bibcode: 2017PLoSO..1282794C.
- ↑ Kurnik, Daniel; Lubetsky, Aharon; Loebstein, Ronen; Almog, Shlomo; Halkin, Hillel (November 2003). "Multivitamin Supplements May Affect Warfarin Anticoagulation in Susceptible Patients" (in en). Annals of Pharmacotherapy 37 (11): 1603–1606. doi:10.1345/aph.1D102. ISSN 1060-0280. PMID 14565795.
- ↑ Harder, Sebastian; Thürmann, Petra (June 1996). "Clinically Important Drug Interactions with Anticoagulants: An Update" (in en). Clinical Pharmacokinetics 30 (6): 416–444. doi:10.2165/00003088-199630060-00002. ISSN 0312-5963. PMID 8792056.
- ↑ Lippi, Giuseppe; Mattiuzzi, Camilla; Franchini, Massimo (April 2016). "Vegetables intake and venous thromboembolism: a systematic review" (in en). Blood Coagulation & Fibrinolysis 27 (3): 242–245. doi:10.1097/MBC.0000000000000427. ISSN 0957-5235. PMID 27023878.
- ↑ "Avocado: Health Benefits, Uses, Side Effects, Dosage & Interactions" (in en). https://www.rxlist.com/avocado/supplements.htm.
- ↑ Dentali, Francesco; Crowther, Mark; Galli, Matteo; Pomero, Fulvio; Garcia, David; Clark, Nathan; Spadafora, Laura; Witt, Daniel et al. (2016-05-27). "Effect of Vitamin K Intake on the Stability of Treatment with Vitamin K Antagonists: A Systematic Review of the Literature" (in en). Seminars in Thrombosis and Hemostasis 42 (6): 671–681. doi:10.1055/s-0036-1581105. ISSN 0094-6176. PMID 27232386.
- ↑ "Warfarin Uses, Dosage, Side Effects" (in en). https://www.drugs.com/warfarin.html.
- ↑ Sullivan, Dawn M.; Ford, Marjorie A.; Boyden, Thomas W. (1998-08-01). "Grapefruit juice and the response to warfarin" (in en). American Journal of Health-System Pharmacy 55 (15): 1581–1583. doi:10.1093/ajhp/55.15.1581. ISSN 1079-2082. PMID 9706183.
- ↑ Di Nisio, Marcello; van Es, Nick; Büller, Harry R (December 2016). "Deep vein thrombosis and pulmonary embolism" (in en). The Lancet 388 (10063): 3060–3073. doi:10.1016/S0140-6736(16)30514-1. PMID 27375038.
- ↑ Koehl, Jennifer L; Hayes, Bryan D.; Al-Samkari, Hanny; Rosovsky, Rachel (2020-01-23). "A comprehensive evaluation of apixaban in the treatment of venous thromboembolism" (in en). Expert Review of Hematology 13 (2): 155–173. doi:10.1080/17474086.2020.1711731. ISSN 1747-4086. PMID 31958251.
- ↑ 73.0 73.1 American Physical Therapy Association (15 September 2014), "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation (American Physical Therapy Association), http://www.choosingwisely.org/doctor-patient-lists/american-physical-therapy-association/, retrieved 15 September 2014, which cites
- "A meta-analysis of bed rest versus early ambulation in the management of pulmonary embolism, deep vein thrombosis, or both". International Journal of Cardiology 137 (1): 37–41. September 2009. doi:10.1016/j.ijcard.2008.06.020. PMID 18691773.
- "Ambulation after deep vein thrombosis: a systematic review". Physiotherapy Canada 61 (3): 133–40. 2009. doi:10.3138/physio.61.3.133. PMID 20514175.
- ↑ Di Minno, Alessandro; Frigerio, Beatrice; Spadarella, Gaia; Ravani, Alessio; Sansaro, Daniela; Amato, Mauro; Kitzmiller, Joseph P.; Pepi, Mauro et al. (July 2017). "Old and new oral anticoagulants: Food, herbal medicines and drug interactions" (in en). Blood Reviews 31 (4): 193–203. doi:10.1016/j.blre.2017.02.001. PMID 28196633.
- ↑ Almarshad, Feras; Alaklabi, Ali; Bakhsh, Ebtisam; Pathan, Aslam; Almegren, Mosaad (December 2018). "Use of direct oral anticoagulants in daily practice" (in en). American Journal of Blood Research 8 (4): 57–72. PMID 30697449.
- ↑ Verdecchia, Paolo; Angeli, Fabio; Aita, Adolfo; Bartolini, Claudia; Reboldi, Gianpaolo (April 2016). "Why switch from warfarin to NOACs?" (in en). Internal and Emergency Medicine 11 (3): 289–293. doi:10.1007/s11739-016-1411-0. ISSN 1828-0447. PMID 26972708.
- ↑ Diener, Hans-Christoph; Ntaios, George; O'Donnell, Martin; Easton, J. Donald (2018-09-22). "Non-vitamin-K oral anticoagulants (NOACs) for the prevention of secondary stroke" (in en). Expert Opinion on Pharmacotherapy 19 (14): 1597–1602. doi:10.1080/14656566.2018.1515913. ISSN 1465-6566. PMID 30152249.
- ↑ Pol, Derk; Curtis, Claire; Ramkumar, Satish; Bittinger, Logan (April 2019). "NOACs Now Mainstream for the Use of Anticoagulation in Non-Valvular Atrial Fibrillation in Australia" (in en). Heart, Lung and Circulation 28 (4): e40–e42. doi:10.1016/j.hlc.2018.03.010. PMID 29861320.
- ↑ "Anticoagulation in atrial fibrillation: NOAC's the word". Deutsches Ärzteblatt International 110 (31–32): 523–4. August 2013. doi:10.3238/arztebl.2013.0523. PMID 24069072. "Things have changed dramatically with the introduction of the new oral anticoagulants (NOACs) — dabigatran, a factor IIa (thrombin) inhibitor, and the factor Xa inhibitors rivaroxaban and apixaban. Clinical trials have shown them therapeutically superior, or at least non-inferior, to VKAs, with less serious side effects.".
- ↑ Heine, Gunnar H.; Brandenburg, Vincent; Schirmer, Stephan H. (2018-04-27). "Orale Antikoagulation bei chronischer Nierenerkrankung und Vorhofflimmern". Deutsches Ärzteblatt Online 115 (17): 287–294. doi:10.3238/arztebl.2018.0287. ISSN 1866-0452. PMID 29789105.
- ↑ "Prescribing Patterns of Novel Anticoagulants Within a Statewide Multispecialty Practice". American Journal of Pharmacy Benefits 8 (3): 97–102. Jun 21, 2016.
- ↑ "2003 Claude S. Hudson Award address in carbohydrate chemistry. Heparin: structure and activity". Journal of Medicinal Chemistry 46 (13): 2551–64. June 2003. doi:10.1021/jm030176m. PMID 12801218.
- ↑ 83.0 83.1 83.2 "Heparin and anticoagulation". Frontiers in Bioscience 21 (7): 1372–92. June 2016. doi:10.2741/4462. PMID 27100512.
- ↑ "Re-visiting the structure of heparin". Carbohydrate Research 403: 60–8. February 2015. doi:10.1016/j.carres.2014.06.023. PMID 25088334.
- ↑ "Preparation, separation, and conformational analysis of differentially sulfated heparin octasaccharide isomers using ion mobility mass spectrometry". Analytical Chemistry 84 (5): 2416–23. March 2012. doi:10.1021/ac203190k. PMID 22283665.
- ↑ "Antithrombin III for critically ill patients: a systematic review with meta-analysis and trial sequential analysis". Intensive Care Medicine 42 (4): 505–520. April 2016. doi:10.1007/s00134-016-4225-7. PMID 26862016.
- ↑ "Human medicines European public assessment report (EPAR): Pradaxa, dabigatran etexilate, Arthroplasty, Replacement,Venous Thromboembolism, Date of authorisation: 17/03/2008, Revision: 29, Status: Authorised". Case Medical Research. 2019-07-16. doi:10.31525/cmr-1321569. ISSN 2643-4652.
- ↑ "Laboratory testing in patients treated with direct oral anticoagulants: a practical guide for clinicians". Journal of Thrombosis and Haemostasis 16 (2): 209–219. February 2018. doi:10.1111/jth.13912. PMID 29193737. https://lirias.kuleuven.be/handle/123456789/611680.
- ↑ 89.0 89.1 "The Reversal of Bleeding Caused by New Oral Anticoagulants (NOACs): A Systematic Review and Meta-Analysis". Clinical and Applied Thrombosis/Hemostasis 24 (9_suppl): 117S–126S. September 2018. doi:10.1177/1076029618796339. PMID 30176738.
- ↑ "Management of Dental Patients Taking Anticoagulants or Antiplatelet Drugs". Scottish Dental Clinical Effectiveness Programme. August 2015. http://www.sdcep.org.uk/wp-content/uploads/2015/09/SDCEP-Anticoagulants-Guidance.pdf.
- ↑ 91.0 91.1 "Role of the anticoagulant monitoring service in 2018: beyond warfarin". Hematology. American Society of Hematology. Education Program 2018 (1): 348–352. November 2018. doi:10.1182/asheducation-2018.1.348. PMID 30504331.
- ↑ 92.0 92.1 Chen, Ashley; Stecker, Eric; A. Warden, Bruce (2020-06-15). "Direct Oral Anticoagulant Use: A Practical Guide to Common Clinical Challenges". Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease 9 (13): e017559. doi:10.1161/JAHA.120.017559. ISSN 2047-9980. PMID 32538234.
- ↑ "Novel anticoagulants". Heart Matters Magazine. British Heart Foundation. https://www.bhf.org.uk/heart-matters-magazine/medical/drug-cabinet/novel-anticoagulants.
- ↑ Ozaki, Aya F.; Choi, Austin S.; Le, Quan T.; Ko, Dennis T.; Han, Janet K.; Park, Sandy S.; Jackevicius, Cynthia A. (2020). "Real-World Adherence and Persistence to Direct Oral Anticoagulants in Patients With Atrial Fibrillation: A Systematic Review and Meta-Analysis". Circulation: Cardiovascular Quality and Outcomes 13 (3): e005969. doi:10.1161/CIRCOUTCOMES.119.005969. ISSN 1941-7705. PMID 32148102.
- ↑ "Overview of betrixaban and its role in clinical practice". American Journal of Health-System Pharmacy 75 (15): 1095–1102. August 2018. doi:10.2146/ajhp170785. PMID 29941506.
- ↑ "RUBY-1: a randomized, double-blind, placebo-controlled trial of the safety and tolerability of the novel oral factor Xa inhibitor darexaban (YM150) following acute coronary syndrome". European Heart Journal 32 (20): 2541–54. October 2011. doi:10.1093/eurheartj/ehr334. PMID 21878434.
- ↑ Dwyer, Joseph; Walsh, Conor (May 2013). "First Time European Approval for Xarelto in ACS". Decision Resources. http://decisionresources.com/The-Decision-Resources-Blog/May-2013/European-Approval-for-Xarelto-in-ACS-052913.
- ↑ "Direct thrombin inhibitors". The New England Journal of Medicine 353 (10): 1028–40. September 2005. doi:10.1056/NEJMra044440. PMID 16148288. https://pure.uva.nl/ws/files/4020551/45334_205943y.pdf.
- ↑ "Exanta". Ask Dr. Stephan Moll. The Thrombophilia Awareness Project. http://www.fvleiden.org/ask/73.html.
- ↑ "Exanta™ (ximelagatran) Study report summaries". AstraZeneca Clinical Trials. http://www.astrazenecaclinicaltrials.com/article/512052.aspx.
- ↑ "World workshop on oral medicine VII: Direct anticoagulant agents management for invasive oral procedures: A systematic review and meta-analysis". Oral Diseases 25 Suppl 1 (S1): 157–173. June 2019. doi:10.1111/odi.13086. PMID 31140701.
- ↑ "Postoperative bleeding risk of direct oral anticoagulants after oral surgery procedures: a systematic review and meta-analysis". International Journal of Oral and Maxillofacial Surgery 47 (7): 923–932. July 2018. doi:10.1016/j.ijom.2018.03.016. PMID 29627150.
- ↑ "Managing patients taking novel oral anticoagulants (NOAs) in dentistry: a discussion paper on clinical implications". BMC Oral Health 16: 5. January 2016. doi:10.1186/s12903-016-0170-7. PMID 26822674.
- ↑ "The dental patient on oral anticoagulant medication: a literature review". Journal of Oral Rehabilitation 38 (8): 615–33. August 2011. doi:10.1111/j.1365-2842.2010.02184.x. PMID 21073495.
- ↑ "Management recommendations for invasive dental treatment in patients using oral antithrombotic medication, including novel oral anticoagulants". Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology 116 (6): 709–16. December 2013. doi:10.1016/j.oooo.2013.07.026. PMID 24120910.
- ↑ "Post-operative Bleeding Risk in Dental Surgery for Patients on Oral Anticoagulant Therapy: A Meta-analysis of Observational Studies". Frontiers in Pharmacology 8: 58. 2017-02-08. doi:10.3389/fphar.2017.00058. PMID 28228727.
- ↑ "Management of Dental Patients Taking Anticoagulants or Antiplatelet Drugs: Dental Clinical Guidance". https://www.sdcep.org.uk/wp-content/uploads/2015/09/SDCEP-Anticoagulants-Guidance.pdf.
- ↑ 108.0 108.1 "Thrombate III label". http://www.talecris-pi.info/inserts/thrombate.pdf.
- ↑ Center for Biologics Evaluation and Research (24 April 2019). "Fractionated Plasma Products - ATryn". https://www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/ucm134042.htm.
- ↑ 110.0 110.1 "Antithrombin (Recombinant) US Package Insert ATryn for Injection February 3, 2009". https://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/UCM134045.pdf.
- ↑ 111.0 111.1 Tornkvist, Max; Smith, J. Gustav; Labaf, Ashkan (February 2018). "Current evidence of oral anticoagulant reversal: A systematic review" (in en). Thrombosis Research 162: 22–31. doi:10.1016/j.thromres.2017.12.003. PMID 29258056.
- ↑ Hanley, J P (2004-11-01). "Warfarin reversal" (in en). Journal of Clinical Pathology 57 (11): 1132–1139. doi:10.1136/jcp.2003.008904. ISSN 0021-9746. PMID 15509671.
- ↑ Makris, M.; Greaves, M.; Phillips, W. S.; Kitchen, S.; Rosendaal, F. R.; Preston, E. F. (March 1997). "Emergency oral anticoagulant reversal: the relative efficacy of infusions of fresh frozen plasma and clotting factor concentrate on correction of the coagulopathy". Thrombosis and Haemostasis 77 (3): 477–480. doi:10.1055/s-0038-1655992. ISSN 0340-6245. PMID 9065997.
- ↑ Chai-Adisaksopha, Chatree; Hillis, Christopher; Siegal, Deborah M.; Movilla, Ron; Heddle, Nancy; Iorio, Alfonso; Crowther, Mark (September 2016). "Prothrombin complex concentrates versus fresh frozen plasma for warfarin reversal A systematic review and meta-analysis" (in en). Thrombosis and Haemostasis 116 (11): 879–890. doi:10.1160/TH16-04-0266. ISSN 0340-6245. PMID 27488143.
- ↑ Pakraftar, Sam (2014). "Dabigatran etixilate and traumatic brain injury: Evolving anticoagulants require evolving care plans" (in en). World Journal of Clinical Cases 2 (8): 362–6. doi:10.12998/wjcc.v2.i8.362. ISSN 2307-8960. PMID 25133148.
- ↑ Ryn, Joanne van; Stangier, Joachim; Haertter, Sebastian; Liesenfeld, Karl-Heinz; Wienen, Wolfgang; Feuring, Martin; Clemens, Andreas (2010). "Dabigatran etexilate – a novel, reversible, oral direct thrombin inhibitor: Interpretation of coagulation assays and reversal of anticoagulant activity" (in en). Thrombosis and Haemostasis 103 (6): 1116–1127. doi:10.1160/TH09-11-0758. ISSN 0340-6245. PMID 20352166.
- ↑ Siegal, Deborah M.; Curnutte, John T.; Connolly, Stuart J.; Lu, Genmin; Conley, Pamela B.; Wiens, Brian L.; Mathur, Vandana S.; Castillo, Janice et al. (2015-12-17). "Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity" (in en). New England Journal of Medicine 373 (25): 2413–2424. doi:10.1056/NEJMoa1510991. ISSN 0028-4793. PMID 26559317.
- ↑ Connolly, Stuart J.; Milling, Truman J.; Eikelboom, John W.; Gibson, C. Michael; Curnutte, John T.; Gold, Alex; Bronson, Michele D.; Lu, Genmin et al. (2016-09-22). "Andexanet Alfa for Acute Major Bleeding Associated with Factor Xa Inhibitors" (in en). New England Journal of Medicine 375 (12): 1131–1141. doi:10.1056/NEJMoa1607887. ISSN 0028-4793. PMID 27573206.
- ↑ Reed, Mirembe; Tadi, Prasanna; Nicolas, Diala (2020), "Andexanet Alfa", StatPearls (StatPearls Publishing), PMID 30137783, http://www.ncbi.nlm.nih.gov/books/NBK519499/, retrieved 2020-01-23
- ↑ Ansell, Jack E.; Bakhru, Sasha H.; Laulicht, Bryan E.; Steiner, Solomon S.; Grosso, Michael A.; Brown, Karen; Dishy, Victor; Lanz, Hans J. et al. (February 2017). "Single-dose ciraparantag safely and completely reverses anticoagulant effects of edoxaban" (in en). Thrombosis and Haemostasis 117 (2): 238–245. doi:10.1160/TH16-03-0224. ISSN 0340-6245. PMID 27853809.
- ↑ Eerenberg, Elise S.; Kamphuisen, Pieter W.; Sijpkens, Meertien K.; Meijers, Joost C.; Buller, Harry R.; Levi, Marcel (2011-10-04). "Reversal of Rivaroxaban and Dabigatran by Prothrombin Complex Concentrate: A Randomized, Placebo-Controlled, Crossover Study in Healthy Subjects" (in en). Circulation 124 (14): 1573–1579. doi:10.1161/CIRCULATIONAHA.111.029017. ISSN 0009-7322. PMID 21900088.
- ↑ Marlu, Raphael; Hodaj, Enkelejda; Paris, Adeline; Albaladejo, Pierre; Crackowski, Jean; Pernod, Gilles (2012). "Effect of non-specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban: A randomised crossover ex vivo study in healthy volunteers" (in en). Thrombosis and Haemostasis 108 (8): 217–224. doi:10.1160/TH12-03-0179. ISSN 0340-6245. PMID 22627883.
- ↑ "Bethesda unit". Biology Online. http://www.biology-online.org/dictionary/Bethesda_unit.
- ↑ Schumacher, Harold Robert (2000). Handbook of Hematologic Pathology. Informa Health Care. p. 583. ISBN 978-0-8247-0170-3.
- ↑ Norman, Mikaela; Jones, Ian (May 2014). "The shift from fluoride/oxalate to acid citrate/fluoride blood collection tubes for glucose testing — The impact upon patient results". Clinical Biochemistry 47 (7–8): 683–685. doi:10.1016/j.clinbiochem.2014.01.011. PMID 24463230.
- ↑ "Management of Dental Patients Taking Anticoagulants or Antiplatelet Drugs – New guidance from SDCEP | Scottish Dental" (in en-GB). 15 September 2015. https://www.scottishdental.org/management-of-dental-patients-taking-anticoagulants-or-antiplatelet-drugs-new-guidance-from-sdcep/.
- ↑ Fredenburgh, James C; Weitz, Jeffrey I (12 October 2020). "New Anticoagulants: Moving Beyond the Direct Oral Anticoagulants". Journal of Thrombosis and Haemostasis 19 (1): 20–29. doi:10.1111/jth.15126. PMID 33047462.
- ↑ "Milvexian for the Prevention of Venous Thromboembolism". The New England Journal of Medicine 385 (23): 2161–2172. November 2021. doi:10.1056/NEJMoa2113194. PMID 34780683.
External links
- Staying Active and Healthy with Blood Thinners by the Agency for Healthcare Research and Quality
- New oral anticoagulants for stroke prevention in atrial fibrillation
 |
 KSF
KSF